Stability and activity of TG25P phage in control of Aeromonas hydrophila in striped catfish pond water
- Department of Biotechnology, Faculty of Chemical Engineering, Ho Chi Minh City University of Technology, Vietnam National University – Ho Chi Minh City (VNU-HCM), 268 Ly Thuong Kiet St., District 10, HCMC, Vietnam
- Ho Chi Minh City University of Technology, VNU-HCM, Viet Nam
Abstract
Introduction: Striped catfish (Pangasianodon hypohthalmus) is a native freshwater fish species in the Mekong Delta, Vietnam, and significantly contributes to national aqua exports. Currently, however, the sustainable development of striped catfish farming is being affected by bacterial pathogen infections, of which hemorrhagic septicemia caused by Aeromonas hydrophila bacteria is one of the most common diseases.
Methods: In this study, the stability of TG25P and CT45P phages to factors such as temperature, pH, and organic solvents was investigated, with the stability of TG25P being found to be higher than that of CT45P.
Results: The activity of TG25P was retained to approximately 90% and 80% at 37oC and 50oC for 1 h, respectively. Its activity was maintained to greater than 80% at pH 5-9 for 24 h and approximately 90-100% in organic solvents, such as chloroform or diethyl ether, for 1 h. In addition, the stability and activity of TG25P for the control of A. hydrophila in striped catfish pond water was also evaluated for 48 h.
Conclusion: TG25P was found to be highly applicable in the creation of low-cost phage-containing products for the prospective application of phage therapy in prevention and treatment of hemorrhagic septicemia in striped catfish.
Introduction
Striped catfish ( or Vietnamese catfish) is a native freshwater catfish species in the Mekong Delta, Vietnam (MKDVN). Vietnam accounts for 90% of global striped catfish production 1. In 2017, the area of striped catfish farms in the MKDVN was 5,822 ha, with a total striped catfish production of 1.3 million tones and an export value of US $1.8 billion dollars 2. However, the annual cycle of bacterial pathogen infections significantly affects the sustainable development of the striped catfish industry in the region. In 2012, and for the striped catfish segment alone, an area of 2,402 ha was infected with pathogens (http://forum.pangasiusmap.com/threads/quan-ly-dich-benh-tren-ca-tra.3), with one of the most common types being hemorrhagic septicemia caused by .
Usage of antibiotics as a measure for prevention and treatment of hemorrhagic septicemia disease has been commonly used in the region. However, the practice has not been appropriately administered and controlled, which has led to undesirable effects and consequences threatening not only striped catfish industry growth but social and economic development in the region. Inadequate control of antibiotic resistance of on striped catfish farms has also been a significant problem. Quach . (2014) demonstrated a high ratio of antibiotic resistance of isolates in ill striped catfish in the MKDVN, such as 100% for ampicillin, amoxicillin, cephalexin, and trimethoprim/sulfamethoxazole, and 93% for tetracycline 3. Moreover, higher-than-approved-limit antibiotic residuals have also been detected in exported stocks. Thus, many consignments have been rejected by importing markets, such as the US, Russia, Japan, South Korea and Canada. In addition, improper usage of antibiotics can negatively affect the farming biological environment over time.
Due to these adverse impacts, there is an urgent need to develop alternative therapies to antibiotics on fish farms. Bacteriophages (or phages) are viruses which infect only bacteria. They were first discovered by Frederick W. Twort 4. Phage therapy involves the therapeutic use of phages to prevent and treat pathogenic bacterial infections. This therapy has only gained serious attention in the aqua industry in the last 30 years due to the wide spread of antibiotic resistance in bacteria. Phage therapy has shown its efficacy in treatment of bacterial diseases in fish and shellfish (reviewed by Richards, 2014; Doss, 2017 56
In our previous study, some phages to control in stripped catfish were isolated and selected based on latent period, burst size, host receptor, . 7. Two phages (TG25P and CT45P) were demonstrated to have short latent periods (40 and 25 min, respectively), high burst size (79 ± 11.9 and 67 ± 1.4 PFU/cell, respectively), and different host receptors for infection initiation. These phages may be promising for phage therapy to control infection in striped catfish. However, phages are constructed relatively simply with a protein capsid and nucleic genome. Their activity is significantly affected by preservation and environmental conditions. Thus, phage stability should be clarified prior to trials.
In this study, the stability of TG25P and CT45P with respect to temperature, pH, and organic solvents was investigated. Furthermore, the stability and activity of TG25P in control of in striped catfish pond water were also evaluated.
Methods
Temperature stability test
Phage stocks of TG25P and CT45P were prepared against strain A1 8. The stability of each phage at various temperatures (4, 20, 25, 30, 37, and 50 C) was investigated by incubating the phage (~10 PFU mL) at the respective temperatures for 1h 7. The experiment was conducted in triplicate. 91011
pH stability test
To determine the stability of the phages at various pHs, the pH of tryptone soya broth (TSB) was adjusted using either 1M HCl or 1M NaOH to attain solutions with pHs of 3, 4, 5, 6, 7, 8, 9, 10 and 11. Each phage suspension (~10 PFU mL) was mixed with an equal volume of the TSB and incubated at 30°C for 24 h 91011
Organic solvent stability test
To assess the stability of the phages in organic solvents, a volume of each phage (~10 PFU mL) was mixed with an equal volume of appropriate organic solvent (ethanol, chloroform, diethyl ether, SM buffer) and incubated at 30°C for 1 h C, 10,000 × g for 10 min. Phage titer was estimated by serial dilution and the double agar-layer method, as described previously. Phage suspension mixed with Phosphate Buffered Saline (PBS) was used as control. The experiment was conducted in triplicate.910
Challenge test in pond water
Inactivation of A1 cells in a striped catfish pond water sample by TG25P phage was examined. The bacterial culture was shaken at 30C, 120 rpm in TSB until its OD of 0.1 (~10 CFU mL) was achieved. The culture was centrifuged at 10,000 × g, 4C, 5 min to obtain a pellet. The pellet was suspended in the same volume of sterilized pond water. The centrifugation and suspension were repeated to discard residuals of TSB. The final pellet was suspended and serially diluted in sterilized pond water to obtain a bacterial concentration of ~10 CFU mL. The solution was divided into two aliquots in Erlenmeyer flasks, with one aliquot being mixed with TG25P phage lysate at a multiplicity of infection (MOI) of 50 (phage : host), and the other aliquot left blank without phage addition. The mixtures were shaken at 30C, 40 rpm. Sampling was performed at 0.5, 1, 2, 3, 4, 6, 8, 10, 12,… and 48 h. In case of the mixture of host bacteria and phage, each sample was divided into two aliquots. One aliquot was serially diluted and spread onto Trypticase Soy Agar (TSA) to estimate bacterial concentration. To the other aliquot, a drop of chloroform was added, incubated for 2 h, and centrifuged at 10,000 × g, 4C, 5 min. The phage titer was estimated by serial dilution and the double agar-layer method, as described above. In case of no phage addition, the sample was serially diluted and spread onto TSA to estimate bacterial concentration. Another control was similarly prepared by adding the phage into sterilized pond water. The phage titer was estimated by serial dilution and the double agar-layer method, as described previously. The experiment was conducted in triplicate.
Results
Thermal and pH stability of phages
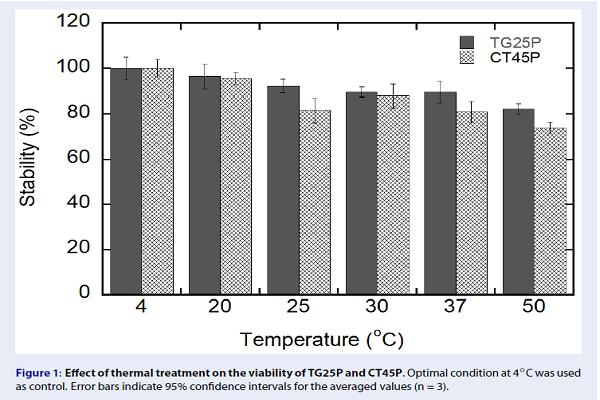
Thermal and pH stability of phages were evaluated. TG25P was found to be relatively thermostable at 20-37 C (Figure 1), with phage activity being retained to approximately 90–95% compared to control. Activity of TG25P was still retained to about 80–85% of the control at the relatively high temperature of 50C. In the case of CT45P, phage activity was relatively similar to that of TG25P at 20, 30, and 37 C. The phage activity of CT45P was approximately 80 and 75% at 25 and 50 C, respectively. The thermal stability of these two phages was much greater than other published phages. Jun (2013) investigated thermal stability of phages against mass mortality of the cyprinid loach () caused by and showed that reduction of their activity was approximately 65 to 79% at 37C, and about 95-98% at 50C 10. Yamaki . (2014) also evaluated the thermal stability of a phage isolated from river water and revealed that about 90% phage activity was lost after 1 h at 50C. Overall, both TG25P and CT45P were thermostable at 20-37C, with TG25P being relatively more stable than CT45P 11.

Effect of thermal treatment on the viability of TG25P and CT45P. Optimal condition at 4°C was used as control. Error bars indicate 95% confidence intervals for the averaged values (n = 3).
A pH stability analysis showed that both phages were stable at pH 5-9, with relatively little difference in the phage titers with respect to control (Figure 2). In contrast, a significant reduction of phage activity was noted at strong acidic (lower than pH 4) and alkaline (higher than pH 10) levels. Both phages presented similar infection capabilities at a pH range of 6-11. CT45P was revealed to be more pH-sensitive than TG25P at pH 4-5. These two phages were more stable in terms of pH than other published phages 1011

Stability of TG25P andCT45P incubated at various pHs. Optimal condition at pH 7 was used as control. Error bars indicate 95% confidence intervals for the averaged values (n = 3).
Organic solvent stability of phage
No effect on phage activity of TG25P was observed after 1 h of incubation with either chloroform or SM (Figure 3). The phage activity was retained to about 65% and 85% after incubation with ethanol or diethyl ether, respectively. TG25P showed high resistance to organic solvents, particularly to ethanol, whereas many other published phages completely lost their activity after treatment with ethanol Figure 3 also demonstrated that TG25P was more resistant to ethanol, chloroform, and diethyl ether than CT45P. Therefore, TG25P was found to be relatively more stable than CT45P in terms of temperature, pH, and organic solvents. This phage was selected to examine its stability and activity in control of in a striped catfish pond water sample.910

Viability of TG25P and CT45P in the presence of various organic solvents. Incubation of phage in sterile PBS was used as control. Error bars indicate 95% confidence intervals for the averaged values (n = 3).
Inactivation of in pond water by phage
Initial host cells at ~10 CFU mL were added into sterilized striped catfish pond water. Figure 4A shows a time course of host cells during the experiment. At the first 2 h of incubation, an increase of host bacterial count was seen for both experiments (with or without phages). However, host bacterial count in the challenge with TG25P sharply decreased in the next 4 h of incubation. This trend was maintained for 8 h of challenge. In contrast, viable bacterial count in the negative control (host cells without phages) maintained the increased trend during the next 8 h and was maintained as stable for 48 h. At 8 h, viable bacterial count of the control was approximately 8.0 log compared to about 5.0 log of the challenge with phages. This result indicated a high-efficient inactivation of in pond water by TG25P when most of the host cells were infected and lysed by phages, resulting in the sharp decrease of bacterial count in the solution. After 8 h, viable bacterial count in the bacterium-phage solution re-increased, indicating growth of phage-resistant bacterial strains. The host cell count was still approximately 1.0 log lower for the bacterium-phage solution than that of the control.

Time course of host cells and phages during the challenge test in striped catfish pond water at 30oC. (A) Bacterial cell count of A. hydrophila in a mixture with TG25P (closed circle) and without phage — negative control (open circle). (B) Phage titer in the mixture with (closed diamond) and without host cells –negative control (open diamond). Error bars indicating 95% confidence intervals for the averaged values (n = 3) are not graphically detectable as the intervals were too narrow.
Figure 4B shows a time course of TG25P phage during the experiment. Together with lysing host cells, phage particles were also newly generated. Phage titer slightly increased during the first 16 h and then sharply increased to 7.5 log until 26 h. The phage titer was then stably maintained. In the control (phage without host cells), phage titer was almost stable during 48 h, indicating the stability of TG25P in striped catfish pond water.
DISCUSSION
is one of the main causative agents of mass mortality in striped catfish in the MKDVN. However, no effective method has been applied to control infection, except for the usage of antibiotics. A high resistance rate of to antibiotics has resulted in a significant loss in production output. Furthermore, antibiotic residuals at higher-than-approved limits have also been detected in exported stocks. The United States is the biggest market for export of Vietnamese striped catfish. However, from August 2, 2017, 100% of consignments of imported striped catfish have been tested for residuals of 89 types of antibiotics by the FDA (2017) 12. Many consignments to leading Vietnamese export countries, such as the US, Japan, South Korea, Canada and Russia, have been rejected due to such antibiotic residuals in the products. Therefore, phage therapy is expected to be an effective solution to replacing antibiotic usage on fish farms in the region since it has shown a high efficacy in treating bacterial diseases in many types of fish and shellfish 1356
Some of the first phages isolated from catfish farms in the MKDVN against were shown in our prospective paper 8. The first trial of phage therapy to treat in striped catfish at a laboratory scale was described by Le . (2018)14. The study preliminarily described efficiency of phage therapy to treat infection in striped catfish. However, the approach of the research had two limitations. First, phages were isolated from Saigon River in Ho Chi Minh City, where no farms of striped catfish were available. Second, the manner of injection of and phage into striped catfish were not realizable at farm scale. To solve both limitations, phage-containing liquid or solid product should be investigated. To apply the phage-containing products at the farm scale, preservation condition of the products should be determined. Preservation of phages has been discussed previously. Generally, most phages maintain their stability when stored at low or freezing temperatures, such as 4, -20, or -70 C. Most research concerned phage preservation in dry or liquid buffer state for usage in the laboratory or medicine 15.
However, phage preservation at ambient temperature is always challenging when the phage concentration decreases sharply in a period of days 16. In the current study, TG25P was found to be quite thermostable, with its activity being maintained to approximately 90% at 37C. In addition, cryopreservatives also significantly support survival of phages 17. TG25P showed a high resistance to organic solvents, such as chloroform, ethanol, and diethyl ether. These organic solvents will protect phage-containing products from contamination of microorganisms. Therefore, TG25P is highly promising in the creation of low-cost phage-containing products stored at ambient temperature.
Striped catfish is relatively vulnerable to pond water conditions, with temperature and pH being two of the most important parameters. Temperature and pH ranges in pond water suitable for striped catfish are 25-32 C 18 and 5.5-9.0 19, respectively. As investigated in the current study, activity of TG25P was maintained at greater than 80% at this temperature and pH ranges. Therefore, this phage represents a highly appropriate antimicrobial agent against on striped catfish farms.
In this study, TG25P also presented a stable phage titer in pond water for 48 h. It showed a high capacity to inactivate growth of in pond water. The study also indicated growth of phage-resistant bacterial strains after 8-h exposure to TG25P phage. The regular emergence of phage-resistant bacteria is one of the major challenges of phage therapy 8. In addition, studies on fixation of phages of fish feed will be also conducted toward application of phage therapy on real striped catfish farms.20212223135
Conclusions
In this study, activity of TG25P phage was demonstrated to be quite stable to different temperatures, pHs and organic solvents such as chloroform, ethanol, and diethyl ether. In addition, it presented a high capacity to inactivate growth of and a stable phage titer in pond water for 48 h. Recently, the sustainable development of striped catfish farming in the Mekong Delta, Vietnam is being affected by hemorrhagic septicemia disease caused by . Therefore, TG25P was found to be highly applicable in creation of low-cost phage-containing products for prospective application of phage therapy in prevention and treatment of hemorrhagic septicemia in striped catfish in the region.
Competing Interests
No conflict of interest declared.
Authors' Contributions
Xuan T.T. Tran implemented the experiment of inactivation of in pond water by phage. Le D. Tam evaluated stability of phage. Hoang A. Hoang proposed the experimental plan and wrote the manuscript.
Acknowledgments
This research is funded by Vietnam National Foundation for Science and Technology Development (NAFOSTED) under grant number 106-NN.04-2015.30; and International Foundation of Science (IFS, Sweden) under grant number I-2-A-5847-2.

