Niosomes: recent advances and applications in targeted drug delivery
- University of Health Sciences, Vietnam National University Ho Chi Minh City
- Vietnam National University Ho Chi Minh City
- Ho Chi Minh City University of Technology, Vietnam National University Ho Chi Minh City
- University of Medicine and Pharmacy at Ho Chi Minh city
Abstract
In drug delivery, niosomes, novel potential biomaterials, were inspired by vesicular surfactant systems. The niosome particles form a bilayered structure containing nonionic surfactants and an aqueous region, allowing them to carry drug molecules with a wide solubility spectrum. Additionally, niosomes provide a cost-effective, biocompatible alternative, ensuring long-term, focused medication and targeted drug delivery. The advantages of niosomes over other drug carrier systems have attracted increasing attention from researchers, who recently published numerous studies on this material as a targeted drug delivery system. Niosomal drugs with high bioavailability can be delivered via various routes of administration, such as oral, dermal, ocular, and respiratory routes. This review presents the fundamentals of the characterization and preparation methods of niosomes. We also highlight recent advancements in the field of targeted drug delivery of niosomes and their applications for routes of administration, highlighting future research trends in niosomes.
Introduction
Currently, addressing drug development related to delivering drugs to their specific sites of action has become increasingly important1. In the early 20 century, Paul Ehrlich introduced the concept of targeted drug delivery and promoted drug delivery system research. The basic principle of this terminology is the delivery of drugs with a sufficient concentration directly to the targeted region, reducing their distribution to the nontargeted area2. Consequently, it produces fewer undesirable effects at other sites and could reduce the amount of drug required to achieve therapeutic effects. In terms of therapeutic agents, a targeted drug delivery system aims to manage and control the pharmacokinetics, pharmacodynamics, specific toxicity, immunogenicity, and biorecognition of drugs3. Therefore, their most crucial application is treating tumor cells because of their better ability to penetrate macrophages, high concentration at the affected site, and fewer side effects4. Furthermore, targeted drug delivery systems have various applications because of their ability to deliver agents via many routes, including ocular, brain, oral, and transdermal routes with diverse carriers and bioactive compounds2, 5.
Direct techniques can be utilized for targeted drug delivery, such as direct injection, catheters, and gene guns, but they are uncomfortable and costly for patients in many cases1. Therefore, the use of specific engineered vectors capable of incorporating drug molecules through encapsulation seems to be an excellent approach6. Numerous novel nanocarriers, including nanoparticles, micelles, nanogels, transferosomes, liposomes, and niosomes, have been developed and applied as drug delivery systems2, 7, 8, 9.
Among these systems, niosomes have recently been acknowledged as promising targeted drugs inspired by lipid-based nanovesicles. Niosomes, with surfactants as their main components, exhibit greater chemical stability and lower production costs despite having a similar structure and utility as other drug delivery vehicles10. Owing to the amphiphilicity of the niosomal structure, hydrophilic molecules can be incorporated into the central cavity, whereas lipophilic molecules can be entrapped within the lipid bilayer. Previous studies have shown that the use of niosomes is a versatile strategy for dermal and transdermal11, nose-to-brain12, oral13, ocular14, and respiratory15 drug delivery.
This review presents the fundamentals of the characterization and updated preparation methods of niosomes. We also provide an overview of the latest advances in the field of targeted niosomal drug carriers for various routes of administration.
Overview of niosomes
Structure
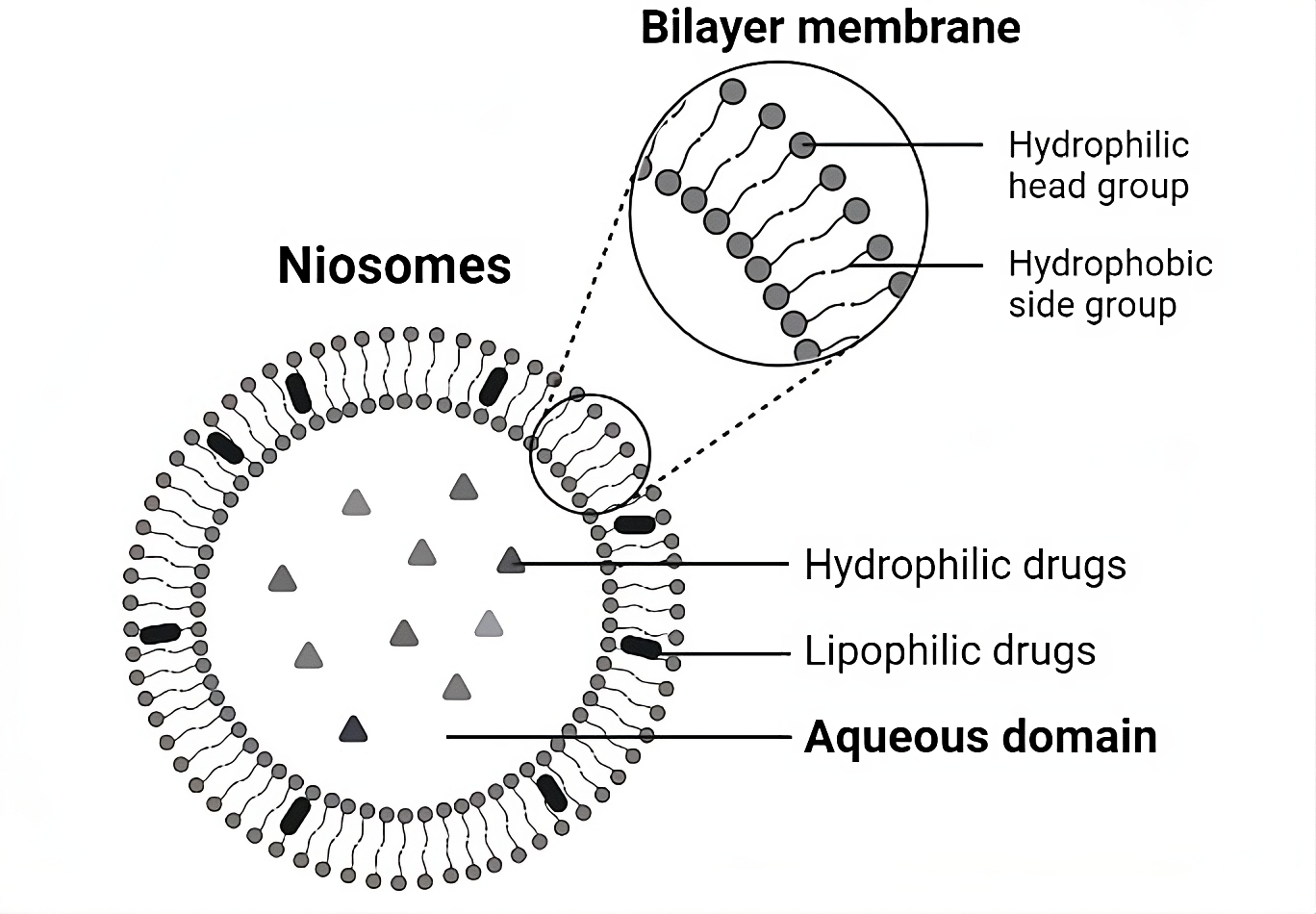
Niosomes are bilayered vesicles composed of polyglycerol alkyl/dialkyl ether nonionic surfactants, such as polysorbate 80 or Span 60, and cholesterol. Nonionic surfactants are amphiphilic molecules with hydrophobic (tails) and hydrophilic (heads) groups that can easily fit into any geometry. Moreover, compared with anionic, cationic, and amphoteric surfactants, nonionic surfactants are more stable and less toxic, enhancing their cellular permeability and solubility. Moreover, cholesterol improves the stability of vesicles by reducing bilayer acyl chain movement through hydrogen bonds between the hydroxyl groups of cholesterol and the alkyl chains of surfactants16. In combination with the type of surfactant, cholesterol significantly affects niosome size. As the cholesterol concentration increases, the hydrophobicity of bilayers increases, decreasing the surface free energy and thereby decreasing the size of niosomes17. The structure of a niosome is shown in Figure 1, which also illustrates the entrapment areas of hydrophilic/lipophilic drugs. The hydrophobic tails remain in contact, whereas the hydrophilic head groups are oriented toward the aqueous interior and exterior of the vesicle. Therefore, with hydrophobic and hydrophilic groups, niosomes can accommodate drug molecules with diverse solubilities8.

The bilayer structure of a niosome.
Types of niosomes
Most categorize niosomes into three basic types on the basis of size and the number of lamellae: small unilamellar vesicles (SUVs), large unilamellar vesicles (LUVs), and multilamellar vesicles (MLVs) (Figure 2)18. Typically, SUVs range in size from 10–100 nm18, LUVs from 100–1000 nm (with the potential to reach up to 5000 nm), and MLVs from 500–1000 nm or without a defined upper limit8, 17, 18. SUVs and LUVs are unilamellar, differing from MLVs, which are multilamellar. The increase in size and number of layers is directly proportional to the number of hydrophilic head groups. In practical terms, this means that SUVs, with their lower aqueous and bilayer ratios than LUVs do, are more suitable for lipophilic drugs, whereas LUVs are better for hydrophilic drugs18, 19. MLVs have bilayers adjacent to aqueous compartments, as shown in Figure 2. Additionally, larger niosomes provide advantages for loading lipophilic drugs because of the presence of multiple bilayers20.

Niosome classification into small unilamellar vesicles (SUVs), large unilamellar vesicles (LUVs), and multilamellar vesicles (MLVs)
Preparation of niosomes
Niosomes can be prepared via various methods to optimize the desired particle size and size distribution, number of bilayers, aqueous phase encapsulation efficiency, and vesicular membrane permeability to transport different drugs, hormones, and antigens21. In thin-film hydration, ether injection, reversed-phase evaporation, or transmembrane pH gradient drug uptake methods, the primary advantages of those methods are easy handling and fast production, which do not require sophisticated equipment. In addition, the thin-film hydration method is a straightforward and convenient technique for obtaining MLVs, and the reverse evaporation method is commonly used to produce LUVs22. The sonication method is the most common size reduction method, which increases drug solubility, enhances bioavailability, and creates niosomes at the nanoscale22. Other novel methods have recently been reported; for example, the microfluidization approach quickly and precisely controls the mixing process of aqueous and surfactant cholesterol solutions in microchannels23. Another form of niosome, the proniosome, is obtained as a dry powder to increase physical stability. The resulting proniosomes are stable throughout storage and transportation and are suitable for making unit dosages24, 25.
Furthermore, the classification of niosome synthesis considers the active and passive trapping approaches by the niosome. The majority of niosome synthesis methods employ the passive trapping approach. This procedure aids in the encapsulation of the medication during niosome synthesis. In contrast, active trapping techniques make it more accessible for the medicine to be absorbed inside the niosome, as the niosome is produced on the basis of an ion gradient or pH gradient. The advantages of this method include cost-effectiveness, high drug–lipid ratios, reduced leakage, and applicability for thermosensitive compounds20. The preparation category, types of vesicle size of each method, and their benefits and drawbacks for a clearer understanding of all the approaches discussed above are displayed in
Advantages and disadvantages of niosome preparation methods.
|
No. |
Method |
Types of vesicle size formed |
Advantages |
Disadvantages |
Preparation category20 |
|
1 |
Thin-film hydration |
MLVs |
Simple laboratory-scale technique. Low polydispersity index (PDI), high stability, suitable for scale-up, good biomolecular film formation |
Utilization of organic solvents. Big-size, multilamellar, low entrapment efficacy (EE) of niosome |
Passive trapping |
|
2 |
Trans-membrane pH gradient drug uptake process |
MLVs |
Easy and simple technique. High drug EE% Good retention of drug |
Utilization of organic solvents. |
Active trapping |
|
3 |
Niosomes prepared through the utilization of micelle solution and enzymes |
MLVs |
Organic solvent-free |
Possible enzymatic degradation of drug molecules |
- |
|
4 |
Sonication |
MLVs |
Simple and easy method. Organic solvent-free, green technique Small particle size, high monodispersity |
Probable titanium particles shedding and substantial increase in temperature due to high energy input |
Passive trapping |
|
5 |
Freeze and thaw |
MLVs |
Simple technique applicable for protein encapsulation Organic solvent-free Easy to scale-up |
Possible leakage of entrapped drug molecules |
- |
|
6 |
Reversed-phase evaporation |
LUVs |
High drug EE% Uniform size and unilamellar or oligolamellar structures of synthesized niosomes |
Utilization of organic solvents. |
Passive trapping |
|
7 |
Bubble |
LUVs |
Organic solvent-free Easy technique |
Not applicable for heat-labile drugs |
Passive trapping |
|
8 |
Supercritical reversed-phase evaporation |
LUVs |
Organic solvent-free One step production and easy to scale up |
Special equipment requirement |
- |
|
9 |
Microfluidization |
LUVs and SUVs |
Organic solvent-free Good reproducibility and ease of formulation |
Not applicable for heat-labile drugs |
Passive trapping |
|
10 |
Ether injection |
LUVs or SUVs |
Simple laboratory-scale technique. |
Not applicable for heat-labile drugs. Utilization of organic solvents. Heterogeneous and large PDI, low EE, toxicity |
Passive trapping |
|
11 |
Heating |
SUVs |
Organic solvent-free |
Not applicable for heat-labile drugs |
- |
|
12 |
Emulsion |
Miscellaneous |
Simple laboratory-scale technique |
Utilization of organic solvents |
- |
|
13 |
Lipid injection method |
Miscellaneous |
Organic solvent-free |
Not applicable for heat-labile drugs |
Passive trapping |
|
14 |
Formation of niosomes from proniosomes |
Miscellaneous |
Organic solvent-free Good physical and chemical stability for long-term storage Convenience for transportation, and ease to scale up |
Complex process Incomplete drug entrapment during hydration |
- |
Although thin film methods, solvent injection, and heating methods are simple and universal, implementing these processes in clinical practice is constrained by several steps, leading to time-consuming and challenging conversion to an industrial scale46, 47. Additionally, the above methods involve complex mechanical and chemical environments for control. The resulting vesicles are often large and polydisperse48; thus, they need an extra step of size reduction by sonication, extrusion or freezing and thawing cycles. However, a novel preparation, called the microfluidization method, can overcome these restrictions and support niosome creation on an industrial scale49, 50.
In recent years, microfluidization has been one of the most commonly employed methods to produce niosomes. By optimizing parameters, such as mixing conditions, the type and ratio of surfactants, and other materials, niosomes with desired particle sizes and size distributions can be produced with better reproducibility than other techniques51, which are based on the jet principle52. In the microfluidization method, the drug and surfactant are mixed and pumped under reservoir pressure into an ice-packed interaction chamber. The generated heat is removed during the passage of the solution through a cooling loop21, 23, 53. Compared with other methods, microfluidization is uniquely superior to conventional modification procedures in some cases, such as continuous rapid processing time54. Furthermore, vesicles of a specific size have been strictly controlled by precisely adjusting the flow rates of the aqueous and organic streams while minimizing the necessary liquid volumes and thereby being able to synthesize multiple types of niosomes simultaneously, reducing preparation time and development costs as well as meeting specific quality requirements8, 50. Additionally, ethanol or isopropanol, which are typically used in this method's solvents, make it more ecologically friendly than other methods are. The microfluidizer is one of several commercially available homogenizers that may produce niosomes on a large scale, hence avoiding intermediary research. However, no studies have described this technique of niosome preparation55. Owing to these benefits, the microfluidization method potentially has a strong chance of being applied at an industrial scale.
Characterization of niosomes
Entrapment efficiency (EE%) and loading efficacy (LE%)
For drug delivery, the crucial parameters of niosomes are entrapment efficiency (EE%) and loading efficacy (LE%)56. The entrapment efficacy (EE%) is the percentage of the drug successfully encapsulated within lipid nanoparticles relative to the total amount of drug used in the formulation process and is aimed at assessing the appropriateness and minimizing the waste of drug usage. In contrast, loading efficacy (LE%) is the ratio of the encapsulated drug to the total weight of the nanoparticles, including both the drug and the lipid carrier, aimed at determining the average drug dosage in each nanoparticle, thereby optimizing distribution efficiency and therapeutic impact57.
The EE% of niosomes depends on the characteristics of drug molecules, surfactants, and additives and the preparation method. In particular, the EE% values of the niosomes obtained via the ether injection method are superior to those obtained via the thin-film hydration method. Adding cholesterol or diacetyl phosphate to nonionic surfactants alters the EE%58. Moreover, the size and number of bilayers affect the choice of loaded drugs and the EE%. In fact, SUVs are less thermodynamically stable, resulting in poor hydrophilic drug loading capacity and easy aggregation. Conversely, LUVs with large aqueous compartments are favorable for hydrophilic drugs. With multiple bilayer membranes, MUVs have a relatively high EE for lipophilic drugs18. The EE% and LE% are calculated via the following formulas:
Several methods have been applied to calculate the EE%, such as spectrophotometry59, 60, 61, 62, 63, 64 and reverse-phase high-performance liquid chromatography65. The purpose of these methods is to quantify the amount of unentrapped drug in the supernatant after centrifugation. When Span is used in formulations, hydrophobic drugs are entrapped more effectively than Tween because the lipophilic part of Span results in more significant space in the bilayer domain65. In addition, the structure of the bilayer, size, and surface charge of niosomes affect the EE%66. Damera’s study showed that a hydrophobic drug (coumarin-153) was entrapped effectively by Span 60-niosomes, whereas Span 80-niosomes had better EE% for a hydrophilic molecule, rhodamine 6G (R6G). Owing to the structural difference between Span 60 and Span 80, the latter contains an unsaturation at the 8 carbon in the chain, resulting in a more disordered bilayer structure. The greater entrapment efficacy of R6G when Span 80 is used may be due to its hydrophilic nature; the R6G molecule can penetrate disordered bilayer areas, allowing it to reach the air‒water interface. This results in leakage and decreased entrapment efficiency in Span 80-niosomes for hydrophobic dyes66.
Size, zeta potential, and stability
During the preparation of niosomes, size and zeta potential (ζ-potential) are two factors that should be monitored carefully. The ζ-potential refers to the potential at the slipping or shear plane of a colloid particle moving under an electric field67, a parameter of particular interest owing to its profound impact on the pharmacokinetics and efficacy of nanomedicine68. Although niosomes can reach sizes of several micrometres, for successful drug encapsulation, good stability, and optimal release kinetics, the particle size should be smaller than 1000 nm40. The particle size can be influenced and adjusted by various factors, such as the synthetic materials and the synthesis process. When selecting components for niosome synthesis, the desired type of particle to be synthesized, the type of surfactant, and the ratio of surfactant to cholesterol are essential to consider. The selection of surfactant is crucial because particle size generally increases with increasing hydrophilic‒lipophilic balance (HLB) index16, which is defined as the ratio of the weight percentages of hydrophilic and lipophilic groups within a molecule69. Similarly, Hedayati demonstrated that the particle size of niosomes prepared via the thin film hydration method depends on the surfactant-to-cholesterol ratio62. Additionally, several methods can be used to reduce the particle size to the desired level. Fatemeh Nowroozi . utilized the thin film hydration method to compare the effectiveness of current size reduction techniques, including sonication, extrusion, and high-pressure homogenization. For niosomes prepared using tween 60 and span 60, the most effective methods for reducing particle size are extrusion and sonication16.
Another significant aspect, the ζ-potential, significantly impacts the behavior and properties of vesicles70. Because of the direct correlation with the repulsion of niosomes, niosomes with largely negative or positive ζ-potentials tend to be prevented from aggregating65. Jain reported that an increase in the HLB value of a surfactant directly correlates with a more negative zeta potential due to higher surface energy and the resulting repulsion of like-charged particles, which inhibits niosomal particle aggregation71. These results can be attributed to the highly hydrophilic HLB, which envelops the niosome in the hydrophilic layer, leading to pronounced negative charges on the surface of the niosome.
Niosome stability is crucial for maintaining the structure of the niosome and preventing drug leakage from its carrier before administration or reaching the targeted site in the body. Niosome stability depends on many factors, such as the nature of the drug molecule, the surfactant properties, the concentration of additives, and the storage conditions. The stability is directly evaluated through the particle size and zeta potential. According to Moazeni , niosomes ranging from 1–10 µm are more stable than those smaller than submicrons. Thermodynamically, smaller niosomes have higher surface free energy and tend to aggregate to minimize excess free energy72. Additionally, MLVs can be easily prepared without complex techniques and offer better stability than can SUVs and LUVs under normal storage conditions. However, it is crucial to consider the desired target effect to determine the appropriate size and to select a suitable surfactant, as mentioned previously. Moreover, particles in the LNP group should have a zeta potential greater than +30 mV or less than -30 mV to indicate electrical stability73. However, in some instances where nonionic surfactants are used, the zeta potential falls within the range of ±30 mV, which does not necessarily mean that the niosome lacks stability. A study by Junyaprasert . revealed that the highest stability is achieved when the zeta potential is less than -30 mV. Nonetheless, samples with zeta potentials lower than this range can still demonstrate stability. Spatial stability enhances the physical stability of niosomes with nonionic surfactants. These findings indicate that electrical and spatial stability are the most critical indicators of niosomal stability, as reflected by the average particle size and entrapment efficacy74. The HLB value is widely recognized as a crucial factor in niosome synthesis, as it is regarded as an indirect determinant influencing the stability of the particles. In addition to these properties, Akbarzadeh performed a stability study under two conditions, at room temperature (25±1 °C) and under refrigeration (4±1 °C) for three months, and the results revealed that the niosome stability was excellent under cold conditions75. A similar experiment was conducted by Suthinee Sangkana to assess the stability of GME-loaded niosomes stored at 4 and 25 °C. The study revealed that niosomes exhibited better stability at 4 °C. Interestingly, GME-loaded niosomes become nearly neutral when stored at 25 °C for 4 weeks, likely due to surfactant degradation, which can disrupt their structure and surface charge. High temperatures can cause surfactants to lose their amphiphilic properties, leading to their degradation. Additionally, vesicle fusion may occur due to increased molecular motion at higher temperatures, causing niosomes to merge and dilute the surface charge76. Similarly, Hashim's findings revealed that the chosen niosomal formulation, which was stable in the refrigerator, had only 2% encapsulated drug leakage at the end of six months61.
and release
The drug concentration, hydration volume, and other factors can impact drug release from niosomal particles. The drug molecules released from the niosomes diffuse through a dialysis membrane at 37 °C in phosphate-buffered saline (pH 7.4 and 5.2)58, 59, 63, 75, 77. In the next step, high-performance liquid chromatography methods are utilized to calculate the amount of drug released59, 65, 75. The drug release process consists of two phases: a fast-release phase at the beginning and a prolonged-release phase60, 65. While the drug released in the initial phase is from the niosome surface, the drug molecules from the core of the particles diffuse out in the second phase. These two phases depend on several factors, such as particle size, drug characteristics, and surfactants60, 65.
The effectiveness of nanoparticle synthesis depends critically on the capacity to manage the features of the synthesized particles, such as the particle size, size distribution, and encapsulation efficiency, as these factors affect the performance78. An increase in encapsulation efficiency increases the size of the network, which is related to physical factors such as the vehicle loading volume capacity79. The half-life of drug carrier systems is significantly affected by their size, as more giant vesicles with short serum half-lives tend to accumulate in some organs, such as the lungs, liver, and spleen. Additionally, niosomes may aggregate due to improper zeta potential, which may have unwanted consequences, such as toxicity and reduced targeting effectiveness38. Size also influences the ability to pass through physiological barriers, such as the blood‒brain barrier80, the blood‒retinal barrier81, and the blood‒placental barrier82. The correlation between nanoparticle size and blood‒brain barrier crossing was shown to be nonmonotonic. Among the polystyrene spherical nanoparticles that range between 100 and 500 nm in size, 200 nm particles can penetrate more effectively than 100 nm or 500 nm particles can83. In addition to the particle size, the size distribution parameters also significantly affect the pharmacokinetics since particles with a broader size distribution have greater liver uptake and shorter blood clearance84.
Functionalization of niosomes enhances targeted delivery
By concentrating drugs at specific sites, surface ligands on niosomes have been developed to increase the efficiency of targeted drug delivery. These ligands facilitate the binding or absorption of niosomes precisely by target cells, thereby increasing the effectiveness of drug delivery to the intended cells. The functional biomolecules frequently employed in the surface modification of niosomes include aptamers, chitosan, folic acid, peptides, and transferrin, as outlined in
Niosome formulations functionalized with various biomolecules
|
Functionalizing biomolecules |
Compositions |
Targeted delivery system |
Efficiency |
Reference |
|
Aptamer |
Doxorubicin and cisplatin encapsulated in niosomes and modified with MUC-1 aptamers |
HeLa and U87 cell lines |
Enhanced cytotoxicity effect on cancer cells compared to their unencapsulated combination. |
|
|
Ethanolic extract of propolis loaded in niosome with Ag85A aptamer surface modification |
|
Specific binding and strong inhibition to |
| |
|
Doxorubicin encapsulated in PEGylated niosomes modified with cell-penetrating peptide and transmembrane glycoprotein MUC1 |
|
Strong cytotoxicity of the MUC1 receptor overexpressed HeLa cells |
| |
|
Chitosan |
Doxorubicin and vincristine coloaded in chitosan-adorned niosome |
Human SKBR3 breast cancer cells |
Inhibited cell migration and increased apoptosis, cell uptake, and endocytosis in human SKBR3 breast cancer cells compared to a single drug or a mixture of drugs. |
|
|
Ursolic acid niosomes with chitosan coating |
HeLa and Huh7it cells |
Chitosan coated in the niosome increased cytotoxicity in HeLa cells but less impacted Huh7it cells compared to the uncoated niosome. |
| |
|
Folic acid |
Curcumin loaded in folic acid-conjugated poly(lactic-co-glycolicacid)-polyethylene glycol nanoniosome |
HeLa229 cervical cancer cells |
Good cellular uptake efficiency |
|
|
Letrozole and curcumin loaded in a folic acid-functionalized niosome |
MCF-7 and MDA-MB-231 breast cancer cells. |
High biocompatibility with HEK-293 healthy cells, while having remarkable inhibitory effects on MCF-7 and MDA-MB-231 breast cancer cells; enhanced the apoptosis rate in both cells. |
| |
|
Cisplatin and doxorubicin coloaded in folic acid-functionalized PEGylated niosomes |
A2780 and MCF-7 cell lines |
Increased the rate of apoptosis and showed a significant inhibition of both cell lines compared to free drugs and a two drugs mixture. |
| |
|
5-FU loaded in folic acid-functionalized PEGylated niosomes |
MCF7 and 4T1 breast cancer cell lines |
More stable and higher entrapment efficiency compared to uncoated niosomes. Increased MCF7 and 4T1 breast cancer cell cytotoxicity, and an increased amount of ROS could lead cancer cells to apoptosis. |
| |
|
Peptide |
Dynorphin-B loaded in niosome, functionalized with N-palmitoylglucosamine |
Blood brain barrier |
Strong pronounced antinociceptive effect on male albino mice model |
|
|
Tenofovir loaded in PEGylated niosome conjugated with TAT peptide |
HeLa cells. single cycle replicable HIV virions (Scr HIV) |
Higher cytotoxicity, lower anti-Scr HIV effect and improved Tenofovir release profile of TAT-niosome compared with the unconjugated niosome. |
| |
|
Doxorubicin loaded in niosome, functionalized with prototypic CendR peptide RPARPAR |
NRP-1-dependent targeting of prostate carcinoma epithelial cell lines (PPC-1 and 22Rv1) |
Significant increase of cytotoxic effect for PPC-1 and 22Rv1 cells compared with unfunctionalized niosomes. |
| |
|
Curcumin loaded in niosome, functionalized amphiphilic peptide |
Chronic myeloid leukemia cells |
Cytotoxicity to chronic myeloid leukemia cell (IC50 of 25 µM), noncytotoxic to human normal fibroblast 3T3 cells. |
| |
|
Transferrin |
Hydroxycamptothecin loaded PEGylated niosomes with transferrin receptor |
Carcinomatous cell lines (KB, K562 and S180 cells) |
Significant toxicity, great intracellular uptake especially in nuclei, high tumor concentration, powerful anti-tumor activity. |
|
|
Doxorubicin hydrochloride loaded in transferrin-conjugated pluronic niosomes |
MCF-7 and MDA-MB-231 breast cancer cells. |
Great extents of cellular uptake by MCF-7 and MDA-MB-231 cells; significant reduction in viability of cancer cells in a dose- and time-related manner. |
|
Routes of administration and applications
Oral administration
Overview
Oral administration is the most common route because of its advantages, including ease of use, patient preference, cost-effectiveness, suitability for repeated and prolonged use, and the lack of sterile precautions needed99, 100. However, drugs are easily degradable through oral administration because of their first-pass metabolism, highly acidic gastric environment, and mucosal enzymes before they enter the systemic circulation101. Numerous drugs may not be absorbed because of inadequate solubility, lipophilicity, and high molecular weight101. Therefore, many scientists have investigated the preparation of orally active substances loaded with niosomes to overcome the limitations of classic oral formulations102.
Niosomes can cross the intestinal membrane, involving paracellular permeation, M-cell-mediated absorption, and endocytosis, as shown in Figure 3. The endocytosis process involves four submechanisms: clathrin-mediated endocytosis, caveolae, micropinocytosis, and phagocytosis103.

Mechanisms of niosome absorption, including A) paracellular permeation, B) M-cell-mediated absorption, and C) endocytosis
Applications
Several compounds have been formulated and innovated to overcome the first-pass metabolism effect and take advantage of oral administration. Coenzyme Q10 (CoQ10) is an endogenous substance with various pharmacological properties. CoQ10 is unstable under light, thermolabile, poorly water soluble, and has low bioavailability; therefore, CoQ10 is entrapped in niosomes coated with polyethylene glycol (PEG) and chitosan (CS) to increase its therapeutic efficacy and improve its release pharmacokinetics. The results revealed that PEG and CS modification significantly increased the hydroxyl radical scavenging capacity of CoQ10. The best PEG coating was 1:2 PEG/surfactant, which significantly increased the stability of the preloaded formulation against light, heat, and storage conditions. Furthermore, the PEG-coated niosomes had more sustained release effects than did the CoQ10 solution in the study in which the dialysis method was used for 24 hours. However, the CS modification caused some adverse effects, such as disturbing the bilayer structure, leading to a leak of CoQ10104.
In addition, the gastrointestinal residence time increased when chitosan was applied as a bioadhesive coating for niosomes105. Owing to the strong mucoadhesive and absorption-enhancing properties of chitosan, encapsulating poorly water-soluble drugs into chitosan-coated niosomes could improve their bioavailability. In a study by AbuElfadl ., candesartan cilexetil was loaded onto a modified-surface niosome, and an increase in bioavailability of 37% was observed106. Another active drug is famotidine, which is also loaded in chitosan-modified niosomal vesicles. Compared with the uncoated formulation, the results demonstrated that 0.1 mg/mL chitosan-coated niosome had greater mucoadhesive effectiveness and stability107.
Niosomes are also used as carriers of antitumor substances. Galangin is a flavonoid compound with antitumor activity that is a promising agent against liver cancer but has poor solubility108. Thus, galangin-loaded niosomes constitute a promising targeted system for enhancing antitumor activity against hepatocarcinoma. Niosomes have longer storage times than liposomes do, while the amount of galangin leaching in the drug release assay increases. Galangin-loaded niosomes effectively decreased the number of neoplastic hepatic lesions with a small number of hepatic adenomas and minichromosome maintenance in three immunostained hepatocytes108. In a study by Sabry ., BiloNiosome-core/chitosan-shell hybrid nanocarriers with cordycepin were studied for their ability to improve oral drug efficacy. The self-association of nonionic surfactants, lipids, and cholesterol produced vesicular nanoparticles to deliver cordycepin. The incorporation of bile salts into these nanocarriers prevents gastrointestinal degradation. Moreover, mucoadhesive chitosan on the surface of the nanoparticles improved their ability to target mucosal surfaces. The results revealed that hybrid nanocarriers were stable in gastric fluid, mucoadhesive, and permeable across intestinal epithelium models and had greater anticancer effects than did the standard cordycepin109. Q. Wang produced ginsenoside Rb1-loaded niosomes via response surface methodology and a central composite design tool to increase its oral bioavailability. Notably, the resulting niosomes showed an increase in oral bioavailability of 181.93% (p < 0.05) compared with the free ginsenoside Rb1110.
In addition, proniosomes are dehydrated forms of niosomes and should be soaked before usage to produce an aqueous niosome dispersion45. In these dry forms, proniosomes have some benefits over niosomes in terms of aggregation, fusion, caking, transportation and distribution111. According to Ibrahim and Shehata, the application of tramadol HCl-loaded niosomes via proniosome technology could help prolong the pharmacological effects of niosomes compared with those of the solution in mice. The improved efficacy of tramadol HCl is due to an increase in the hydrophilic‒lipophilic balance of the surfactant. Drug release increased as the cholesterol content increased from 0% to 50%. At 10% cholesterol, drug release increased in a specific order: Span 80 > Span 40 > Tween 80 > Tween 40112.
Advantages and disadvantages
As a drug delivery vehicle via the oral route, the advantages of niosomal formulations include improved stability and bioavailability113. Both lipophilic and hydrophilic drugs can be formulated easily and combined in the same vesicles114. Coating vesicular systems protects delicate molecules, increasing their bioavailability through the intestine. Nonionic surfactants such as Span, Tween, Brij, and poloxamer are permeation enhancers that increase the ability of drugs to permeate through intestinal tissue115. Niosomes may significantly extend the half-life of drug molecules in the plasma, making it possible to minimize the frequency of drug administration116. A study by Kamboj on niosomes loaded with tenofovir disoproxil fumarate, an anti-HIV drug, demonstrated a significant increase in the mean residence time and more than a twofold increase in oral bioavailability compared with the same dose of the drug in its standard form116. The release of niosomes can be controlled or sustained in transit and at the localization site61. The stability of nanocarriers under gastrointestinal conditions is a serious concern, influencing the therapeutic efficacy of the formulation117. In the future, niosomes can be engineered to transport drug molecules to desired locations by surface modification via the attachment of various ligands or magnetic guidance.
Transdermal delivery
Overview
The topical application of niosomes can improve drug penetration into the epidermis and dermis, as reported by many previous studies118, 119, 120, 121, 122, 123. The following mechanisms explain this effect. First, when drug-containing niosomes are absorbed on the skin surface, a high degree of drug thermodynamic activity occurs at the vesicle and stratum corneum (SC) surfaces, which facilitates the penetration of drugs through the SC124, 125, 126, 127, 128. Second, niosomal formulations disrupt the densely packed lipids that fill the extracellular space of the SC, enhancing drug permeability through conformational alteration of the SC. Third, transdermal penetration is improved in the presence of nonionic surfactants as penetration enhancers, which alter the intercellular lipids of the SC and increase the overall membrane fluidity24, 124, 125, 127, 129, 130, 131, 132, 133, 134, 135. Finally, niosomes induce a hydrating effect, which loosens the tight cellular arrangement of the SC124, 127. The dermal and transdermal routes of delivery of niosomes are briefly presented in Figure 4.

Skin penetration pathway of niosomes and proniosomes in dermal and transdermal drug delivery. (A) Niosomes release drug particles. (B) Niosomes disrupt the densely packed lipids that fill the extracellular space of the SC. (C) Niosomes disrupt their structure, and niosomal fragments and drug particles diffuse through the SC extracellular space. (D) Niosomal surfactants act as penetration enhancers and increase membrane fluidity. (E) Niosomes diffuse through hair follicles.
Applications
Since 1975, many dermatological products applying niosome technology, such as the antiaging Lancôme Niosome Plus, have been developed and put on the market136. Current efforts focus on the clinical application of niosomes as local drug delivery systems137.
Local anesthesia
Before performing skin procedures, dermatologists take advantage of the benefits of local anesthetics for pain relief. Ineffective local anesthetic formulations can lead to inadequate anesthesia, severe dermatitis, and systemic toxicity138. The importance of the delivery system and its surfactants was demonstrated by Shabery semisolid niosomal encapsulated lidocaine and prilocaine were prepared via a mixture of Span 40 or Span 60, cholesterol, and a patented palm oil base. The results showed that among the formulations, formulation F1 containing Span 40 had the greatest EE%, as well as the highest permeability, of the two anesthetics. Furthermore, the formulation of the F1-C emulsion was optimized via a cold process, resulting in an even higher permeability rate. This led to a strong anesthetic effect at the administration site. Compared with the EMLA cream, the F1-C emulgel had a comparable local anesthetic effect on healthy subjects139.
Psoriasis
Psoriasis is a chronic systemic inflammation of the skin that causes discomfort, pain, itch and disfiguring skin lesions, which can trigger the psychosocial burden of the disease140. The effectiveness and safety of antipsoriatic treatments could be improved through the use of topical niosomal formulations. Methotrexate (MTX) is an antifolate used to treat psoriasis that causes many side effects, particularly hepatotoxicity, when it is systemically administered141. Dermal delivery of MTX could be a promising strategy to avoid systemic side effects142. Lakshmi prepared MTX-loaded niosomes in a chitosan gel, and the clinical evaluation results revealed that this niosomal system was more effective than the placebo and marketed MTX gels143. In addition, niosomes encapsulating celastrol, a triterpenoid found in the genus Tripterygium, significantly improved its antipsoriatic activity in rats by increasing its water solubility and skin penetration144.
Vitiligo
Vitiligo is caused by locally damaged melanocytes, resulting in white, discolored areas of the skin. Although vitiligo is not a life-threatening problem, it harms quality of life145. The application of transdermal drug delivery systems could play an essential role in overcoming the drawbacks of conventional topical formulations in terms of efficacy and safety146. Defects in human tyrosinase, an enzyme involved in melanogenesis, lead to the loss or inactivity of melanocytes and have been demonstrated to be one of the causes of vitiligo or depigmented skin147, 148. Elastic cationic niosomes, including Tween 61/cholesterol/dimethyl dioctadecyl ammonium bromide with a molar ratio of 1:1:0.5, were studied by Manosroi (2010) for efficient transdermal delivery of pMEL34 (tyrosinase-encoding plasmid). The results showed that this system produced better permeability flux in the viable epidermis and dermis than inelastic niosomes did149. By applying elastic cationic niosomes loaded with pMEL34 in melanoma cell lines, tyrosinase gene expression was approximately four times higher than that of free plasmids and pMEL34 loaded in inelastic niosomes149. In a study by Manosroi . (2009), luciferase plasmids were enclosed in elastic and inelastic cationic niosomes, creating a novel method for the transdermal delivery of genetic material in gene therapy via electrophoresis150. In particular, elastic cationic niosomes are promising because they do not require additional equipment. This study revealed the superiority of niosomes in their ability to deliver luciferase plasmids through the skin150. Both studies indicated that elastic cationic niosomes can effectively transport genetic materials for local gene therapy.
Cutaneous inflammation
Celecoxib, a selective cyclooxygenase-2 inhibitor, was encapsulated in a niosomal gel for site-specific retention and delivery. Auda . prepared gels with Span 60 or Span 40 and cholesterol using different polymers as bases. As a result, the formula with a 1:1 ratio of Sponge 60:cholesterol resulted in the highest encapsulation and significantly increased the drug's release (to 80%). The anti-inflammatory activity of the gel formulations was investigated, and the niosomal poloxamer gel significantly reduced rat paw edema (75.4%), resulting in better permeation flux. The results revealed that niosomal gel formulations made with polymers are suitable carriers for skin accumulation and anti-inflammatory effects151. Nasr . employed thin film hydration to create multilamellar liposomes and niosomes with aceclofenac, a potent analgesic, antipyretic, and anti-inflammatory agent. Compared with commercial products, both systems were shown to prolong the pharmacological effect of the encapsulated drugs151. In particular, the efficacy of niosomes outperformed that of liposomes in animal models152. In the studies by Bhardwaj and Bhatia, Tweens and the natural mucilage of were combined to create niosome gel-loaded ibuprofen. With increasing concentrations of, the results demonstrated a reduction in drug permeation and drug flux values. These findings suggest that mucilage has good binding ability and thus regulates the release of ibuprofen from the niosomal gel system. and tests revealed that niosomal gel formulations combined with carbopol and had superior penetration and efficacy than carbopol formulations alone. This may be due to better skin retention and deposition, which may have increased drug partitioning into the rat paw and prolonged and boosted its anti-inflammatory effect153.
Topical corticosteroids are used for various dermatological disorders because corticosteroids with an appropriate carrier can prolong their effect, thereby reducing the frequency of doses and associated side effects. In a study by Sun Zhe, clobetasol propionate, a potent anti-inflammatory and antipruritic agent, was incorporated into a niosomal gel. The performance of the niosomal clobetasol gel was compared to commercially available gels, considering drug deposition in the stratum corneum (SC) layer and studies. The findings revealed that the niosomal gel formulation exhibited superior efficacy, reducing the psoriasis area severity index154.
Chlorpheniramine maleate is an antihistamine commonly used topically to treat sunburn, urticaria, angioedema, itching, and insect bites. The proniosomes composed of Span 40/lecithin/cholesterol prepared with ethanol exhibited ideal stability, encapsulation efficiency, particle size, and release kinetics, making them well suited for the transdermal delivery of chlorpheniramine maleate. The simple preparation and ease of use of proniosomes are the most significant advantages this dosage form offers155. Afreen . assessed a niosome-based gel containing chlorpheniramine for treating mild to moderate skin allergies. The optimal niosome dispersion with low cholesterol and span-80 and a high level of span-60 demonstrated a high entrapment efficiency value and maximum drug release. The entrapment efficiency is influenced by the amount of cholesterol and nonionic surfactants, which affects the hydrophobicity, rigidity, and stability of the niosomal layer156.
Alopecia
Hormonal alopecia (male pattern alopecia) is the most common cause of hair loss in men157. Pumpkin seed oil contains β-sitosterol, which has been shown to prevent hair loss by inhibiting 5α-reductase type 1 (a major enzyme responsible for increased dihydrotestosterone production in male patients with hormonal alopecia)158. Research by Teeranachaideekul . revealed that hair loss significantly decreased by 44.42% in a 60-second hair count experiment after eight weeks of pumpkin seed oil-loaded niosomes159. Finasteride, a 5α-reductase inhibitor, has several undesirable systemic side effects that can be alleviated if it acts locally in hair follicles160. Finasteride-loaded proniosomes (FLP) greatly increased the quantity and size of hair follicles in the dorsal areas of C57BL/6MLac mice. In addition, although the concentration of finasteride in FLP (1%) was lower than that in the minoxidil solution used (2%), the therapeutic effects of these two treatments were comparable. Accordingly, the results showed that FLP might be utilized to prevent hair loss by inhibiting type I 5α-reductase161. Minoxidil is the most commonly used drug to treat male hormonal alopecia162, 163. Mali . reported that Span 60 created stable niosomes with the highest entrapment effectiveness (31.27±1.5%) and the smallest particle size. Compared with those of conventional minoxidil gels, the permeability and deposition of minoxidil in niosomal gels were significantly improved162.
Acne
Acne is a prevalent skin disorder during adolescence. Treatment options often involve topical analgesics, although their side effects might compromise efficacy and patient satisfaction. Niosomes with excellent potential for dermal administration may help improve the transdermal delivery of antacid agents by facilitating their local accumulation with reduced side effects164. Benzoyl peroxide, a first-line acne treatment with poor water solubility, the formation of larger clumps, and modest dermal penetration when applied topically, necessitates high amounts of the drug to achieve the desired effect, resulting in skin irritation. Gagan Goyal developed a carbopol niosomal gel formulation containing benzoyl peroxide, which significantly improved the skin penetration of the active ingredient. Compared with conventional medicine and a simple niosomal formulation, the application of niosomal gel had a significant antibacterial effect after four days of treatment, reducing skin inflammation and irritation165. Manosroi developed an anionic niosome formulation loaded with gallidermin formed from Tween 61, cholesterol, and diacetylphosphate. The results from the absorption through rat skin revealed that the accumulation in the epidermis and dermis of the rat skin was more than two times greater than that of an aqueous solution of gallidermin; furthermore, gallidermin loaded in niosomes at high temperatures exhibited better chemical stability166. This research suggests the potential of the anionic niosomes of gallidermin as effective topical antibacterial formulations without the risk of systemic effects166. The percutaneous delivery of tretinoin-loaded niosomal formulations across neonatal pig skin was investigated via Franz diffusion cells. The results from these assays revealed better skin retention than that of liposomal and commercial formulations167. Rosemarinic acid is a natural ester of caffeic acid with antibacterial and anti-inflammatory properties. Rosemarinic acid-loaded niosomes have been reported to deliver biological antibacterial agents into deeper layers of the skin168. A clinical study was also performed with niosomes loaded with retinoids, critical ingredients in topical acne treatment. The results showed that niosomal retinoids improved antiacne efficacy and were safe for long-term use168. Recently, the development of new antibiotics for treating acne has attracted considerable interest. For skin delivery, various solid forms of roxithromycin are packaged into vesicular systems (niosomes, proniosomes, ufosomes, and pro-ufosomes). In particular, niosomes are the most effective formulation for drug delivery to the dermis, where they need to be active against bacteria169.
Advantages and disadvantages
Niosomal formulations have been proven to be well-controlled and advantageous drug delivery systems for topical and transdermal administration. Topical niosomes provide stable dosage forms and cause minimal systemic side effects, as drug release is localized at the application site170, 171. Transdermal drug formulations can deliver active ingredients to the systemic circulation. Remarkably, drug delivery via this route is noninvasive (no needles are needed), has relatively high bioavailability because first-pass metabolism and acidic/enzymatic breakdown in the gastrointestinal tract can be avoided, eliminating food‒drug interactions17. Vesicular carriers can also help overcome the physical and chemical instability of some drug molecules. Niosomes, which are more stable and permeable, appear to be a preferred drug delivery system over liposomes137.
In addition to the multiple benefits of transdermal routes, the absorption of drugs could be limited by the transdermal barrier, such as the stratum corneum, and its protective nature172, 173. Ideal drug molecules for transdermal formulations often have small molecular weights (under 500 Da) and high hydrophobicity and preferably have low effective doses166. Consequently, only a limited number of drugs can satisfy these criteria. Furthermore, the liquid nature of the niosomal preparations is unfavorable for topical application17.
Ocular delivery
Overview
The eye is a crucial human sense organ; therefore, any ocular disorder can lead to profound impairment. Prevalent ocular conditions include glaucoma, keratitis, genetic disorders, and diabetic macular edema174, all of which have the potential to impact vision detrimentally and, if not promptly treated, even lead to blindness. Thus, ocular disease treatment is essential and should be prioritized. Nevertheless, the complex structure of the eye, which has numerous barriers and defense mechanisms, leads to several drug delivery challenges. However, antibiotic-loaded niosomes can effectively contact the corneal surface and then release drugs, as shown in Figure 5. In this case, by removing the mucus layer, the surfactants enhance the penetration of antibiotics.

Pathway of drug release from antibiotic-loaded niosomes at the site of action for ocular delivery.
Applications
The common forms used for treating ocular diseases include topical eye drops and ointments175, with the cornea serving as the primary pathway for drug administration to the anterior segment of the eye14. The drug delivery efficiency through this route mainly depends on the molecular weight, lipophilicity, and diffusion of the drugs. However, this drug delivery route still has several disadvantages, such as low bioavailability and challenges in permeability retention time176. Additionally, owing to insufficient time to settle in the conjunctiva, many drugs are washed away from the ocular surface into the nasolacrimal ducts or lacrimal glands. Intravenous and intravitreal administrations are the primary routes for delivery to the posterior part of the eye177. In the clinic, the time interval between injections is usually very long, making it difficult to maintain patient compliance and reducing the efficacy of treatment. Therefore, developing novel ocular drug delivery systems is essential for increasing drug residence time, permeability, and bioavailability.
Glaucoma
In recent years, numerous studies have highlighted the significant potential of niosomes in drug delivery for ocular disease treatment. Glaucoma, a chronic and progressive disorder, often requires prolonged treatment178. The most common antiglaucoma drugs are prostaglandin analogs, beta blockers, carbonic anhydrase inhibitors, adrenergic agonists, and miotics178. Hasan designed and developed a formulation of niosomes loaded with dorzolamide hydrochloride for glaucoma treatment. The results showed that this formulation has a high drug EE% with small niosomes and a longer drug release rate than conventional preparations do179. Jain . developed a formulation of niosomal gel containing pilocarpine hydrochloride to increase the retention time and improve the bioavailability of this antiglaucoma drug. The results revealed that this niosomal gel has a bioavailability 2.64 times greater than that of the commercial pilopine HS gel71. Fathalla . formulated niosomes containing latanoprost, a highly effective drug for glaucoma therapy180. The latanoprost niosomal gel showed a remarkable ability to maintain drug release. Additionally, the results from experiments indicated that the gel has no toxic or irritant effects and reduces intraocular pressure after treating rabbits' eyes for three days180. Recently, Abdelmonem . developed a niosomal formulation comprising acetazolamide and carvedilol to limit their side effects181. Within just one hour after administration, the sustained gel formula of the combination normalized the intraocular pressure, which continued to stabilize for four days. Moreover, histological studies of glaucomatous eyeballs from treated rabbits revealed improvements in retinal atrophy. In addition, other antiglaucoma drugs, such as dorzolamide hydrochloride or brimonidine tartrate, have also been incorporated into proniosome gels, which induce sustained drug action after reconstitution in water182, 183.
Fungal keratitis
Fungal keratitis or keratomycosis is a severe corneal infection caused by corneal trauma, chronic ocular surface disease, topical corticosteroids, or contact lenses184. Fluconazole, voriconazole, natamycin, or ketoconazole preparations are antifungal drugs often used to treat keratomycoses; however, the bioavailability of these drugs is a significant challenge. El-Emam . successfully developed the voriconazole-loaded proniosomal formula, which has a high capture efficiency (87.4±2.55%)185. In an experiment against and , the formulation of proniosomes exhibited excellent antifungal effects and stable drug release. Other antifungal drugs for fungal keratitis treatment have also been studied for their ability to increase bioavailability. Soliman . reported that a niosomal gel containing fluconazole was twice as bioavailable as the corresponding microemulsion186. A study on fluconazole loaded in niosomes for ocular drug delivery was performed by Elmotasem . with the FL-HP-β-CD complex enclosed in eudragit nanoparticles. The results showed that niosomes coated with cationic chitosan improved adhesion, increased corneal permeability, and prolonged action187. As reported by Abdelbary ., ketoconazole-loaded proniosomal gels presented increased corneal exposure, penetration, and ocular retention times, resulting in a sustained effect and increased bioavailability by 20 times compared with that of the conventional suspension188.
Bacterial keratitis
The treatment of bacterial keratitis involves the topical use of antibiotics in combination with corticosteroids to improve patient compliance189. Niosomes containing ethanol and cholesterol have been studied for the intraocular delivery of prednisolone acetate and prednisolone sodium phosphate, and the results have shown promise in drug delivery190. Abdelbary reported that the formulation of lomefloxacin HCl (LXN) in the niosomal form resulted in an increase of 166% in drug permeation across the cornea compared with that of an LXN solution191. Khalil also studied niosomes with LXN on an optimized formula that remained stable under refrigerated conditions for up to three months, and there was a significantly greater percentage of inhibition of than that of a commercial product192. Clinical observations and colony counts of infected eyes revealed considerable improvement in the treatment response. In infected rabbit eyes, the effectiveness of the niosomal formula was evaluated compared with that of a commercial drug. After eight days, the group treated with the LXN-vesicular formula presented no viable bacteria or any signs of inflammation, indicating an improvement in the treatment response. Thus, the niosomal dispersion of LXN could serve as a superior ocular delivery system that holds promise for treating bacterial conjunctivitis192. Further research by Khalil . was carried out to improve the drug bioavailability of LXN in proniosomes193. The optimized proniosomal gel formulation (P-LXN 7) appeared as spherical vesicles with high adhesion efficiency (> 80%) and exhibited controlled drug release for 12 h. P-LXN 7 was shown to be safe and suitable for ophthalmic use, having a greater antibacterial effect than commercially available LXN eye drops. Allam developed a vancomycin-loaded niosomal formulation with high drug capture efficiency and increased eye residence time194. An study on MRSA-infected rabbits revealed that this formulation was 2.5 times more effective in terms of antibacterial activity than a vancomycin-free drug solution194. Gugleva . studied doxycycline-loaded niosomes, which demonstrated sustained release properties and satisfactory stability after two months of storage at 4 °C195. Further research on doxycycline niosomal topical heat-sensing gels also revealed enhanced antibacterial activity compared with that of a doxycycline solution196. To improve the corneal residence time of ciprofloxacin (an antibiotic commonly used to treat bacterial conjunctivitis), Ameeduzzafar developed a formulation of chitosan-coated niosomes loaded with the drug. Compared with commercially available ciprofloxacin eye drops, the optimized formula resulted in a 1.79-fold increase in corneal permeability197. Chitosan-coated niosomes were found to be promising drug transporters for treating bacterial conjunctivitis, as they significantly inhibited bacterial growth and increased the corneal contact time. A niosomal formulation loaded with flurbiprofen to increase the ocular drug retention time and improve its anti-inflammatory activity was reported by El-Saved . Later, and studies revealed reduced drug crystallinity, higher interaction rates with other niosomal contents, and elevated ocular bioavailability than the corresponding flurbiprofen ophthalmic solutions198. Recently, curcumin has been shown to replace common active anti-inflammatory ingredients for the treatment of eye inflammation. The curcumin-loaded PG formulation exhibited a high capture efficiency (96.0 ± 0.1%). The resulting permeability was 3.22 times and 1.76 times greater than that of the dispersed and lyophilized forms, respectively199.
Other niosomal formulations have also been developed to deliver topical medications to the eye. Studies carried out by Puras . revealed that when protamine was incorporated into niosomal DNA vectors, the transformation efficiency, cell viability, and DNA concentration improved. This study highlighted the enhanced properties of protamine/DNA/niosome tertiary vectors for efficient and safe gene delivery to the mouse retina, which could become a new promising genetic material delivery system for the retina200. The azithromycin chitosan-coated niosomes constructed and optimized by Eid . presented a novel colloidal system that increased the residence time, ocular permeability, and bioavailability. The optimized niosomes markedly improved the apparent osmolarity, corneal permeability, and azithromycin concentration in rabbit eyes without significant irritant effects201.
Advantages and disadvantages
As reported in many studies, various forms of niosomes have shown outstanding abilities in ocular drug delivery. They can be considered promising drug carriers for treating different eye conditions. They were demonstrated to enhance the chemical stability, bioavailability, and permeability of drug molecules while reducing side effects. Additionally, nanosize niosomes have been reported to resist drainage due to reflex tearing and blinking; thus, they can be better retained and widely spread on the eye surface than other carriers202. However, more studies on niosomal formulations should be carried out before their commercialization as ophthalmic medicines. It has been reported that niosomes cannot maintain stability under ambient conditions, leading to drug leakage and decreased encapsulation efficiency203, 204. Moreover, targeted drug delivery strategies to the site of action of the eye should be integrated into niosomal formulations to increase the therapeutic effect. In addition, investigating the cytotoxicity and other side effects is essential for the preclinical or clinical stages to ensure the safety of niosomal drugs.
Respiratory delivery
Overview
Recently, the delivery of drugs through the respiratory system has been shown to have both local and systemic effects. Additionally, this application site can offer an attractive, noninvasive route of administration205. Pulmonary drug delivery therapies are widely used to treat respiratory diseases such as inflammation, asthma, chronic obstructive pulmonary disease, cystic fibrosis, diabetes, and other systemic diseases206. The advantages of pulmonary drug delivery include a relatively high degree of convenience and excellent absorption (air-to-blood delivery) because of the large surface area of the lung (i.e., 100 m) and the thin epithelial layer (0.2--0.7 μm)207. On the other hand, local pulmonary drug delivery to treat lung diseases could reduce systemic distribution and adverse effects208.
However, this route of administration also has several limitations, such as the low effectiveness of inhalation systems, the small amount of drug delivered per puff, and the instability of some drugs in these pulmonary formulations58. To reach the lower respiratory tract, the particle size should be between 1 and 5 μm. Particles smaller than 1 μm cannot be retained in the lungs, whereas those larger than 1 μm are deposited mainly in the upper respiratory tract209. In particular, those ranging from 2–5 μm are deposited in the bronchioles by sedimentation, whereas particles less than 2 μm are deposited in the alveoli by Brownian diffusion, as illustrated in Figure 6. Notably, the various transportation mechanisms involved in drug release and absorption across the epithelium depend on the position of the deposition.

Pathway of drug release from niosomes in respiratory drug delivery.
Applications
Gemcitabine (Gem) and cisplatin (Cis) are common drugs used for lung cancer treatment. A study by Mohamad Saimi developed an aerosolized niosome formulation containing Gem and Cis, aiming to reduce the number of dogs due to the targeted capability of the niosome. The authors determined a suitable optimized niosome formulation with an aerosol output of 96.22%. Cytotoxicity to normal lung MRC5 and cancerous A549 pulmonary cell lines was detected, and the results revealed moderate toxicity to A549 cells, with an IC = 46.0 µg/mL, but weak toxicity to MCR5 cells (IC = 280.0 µg/mL). Therefore, aerosolized niosome formulations are promising candidates for aerosolized delivery methods to treat lung cancer with fewer side effects on normal lung cells210. Nintedanib has been used to treat idiopathic pulmonary fibrosis and other lung diseases211. In the study of Shukla ., nintedanib was formulated as an inhalable agent for non-small cell lung cancer treatment. Cytotoxicity studies have indicated that niosomal formulations have greater cytotoxic effects on lung cancer cells (A549, H2122, H1299, H358, and H460) than plain nintedanib does212. Curcumin, a natural phenolic compound isolated from turmeric, shows promise as a chemopreventive and anticancer agent, with multiple studies suggesting its ability to inhibit the onset and improvement of non-small cell lung carcinoma213, 214. Curcumin-loaded niosomes were shown to have anti-inflammatory effects on respiratory syncytial virus-induced respiratory disease in a BALB/c mouse model. The mice were treated daily with the niosome, and after five injections, the lung pathology was alleviated, which reduced immune cell infiltration and the synthesis of inflammatory mediators (MIP-1, TNF-α, and IFN-γ) in the lungs215.
Advantages and disadvantages
Pulmonary drug absorption benefits from an extensive contact area facilitated by the dense capillary network in the alveoli, enhancing the interaction between air and blood216. Niosomal drugs can help improve permeation via pulmonary administration via airway mucus, and these vesicles were also found to significantly increase drug uptake by human lung fibroblasts217. Moreover, they can provide a tangible strategy to resolve formulation-related problems associated with many poorly soluble therapeutic molecules to treat local respiratory and systemic diseases. The niosomal delivery of a drug molecule through the pulmonary route extends its residence time in the lung, preventing systemic side effects. However, limitations exist regarding drug irritation, toxicity, and stability; ensuring drug transport to the intended site of action is not guaranteed, and challenges may arise in drug retention and clearance owing to the efficient clearance mechanisms of the lungs216, 218.
Discussion
During the past decade, with remarkable efficiency and safety as drug carriers, several lipid-based nanoparticles, such as AmBisome® (Amphotericin B), DaunoXome® (daunorubicin), and Doxyl® (doxorubicin), have been successfully commercialized219. Because lipid-based particles are similar in structure to the phospholipid bilayer in cell membranes220, the promise of lipid-based carriers is enormous, leading to the first appearance of liposomes. Since then, an increasing number of different types of lipid particles, such as ethosomes, sphingosomes, transfersomes, and niosomes, whose characteristics have been modified and improved to control drug release and suit different routes of administration. Niosomes offer notable benefits over other lipid nanoparticle varieties, including increased chemical stability, reduced production expenses, abundant nonionic surfactant options, biocompatibility, and biodegradability221, 222.
In addition to some advantages, well-known and popular methods for the preparation of niosomes, such as thin-film hydration, ether injection, and reverse-phase evaporation, involve the use of organic solvents, thereby increasing the risk of their contamination in the final products37. Furthermore, the large particle size and heat sterilization significantly reduce vesicle stability because of their potential to disrupt lipid structures or surfactant-based formulations17. In addition, surfactants play an essential role in the formation of niosomes, determining some properties or limitations of niosomes, such as toxicity, drug release ability, and physicochemical stability223. Numerous papers have recently revealed information about the cytotoxicity of surfactants and niosome molecules224, 225. However, long-term toxicity in animal models after administration has yet to be reported; thus, further studies on tolerability and clinical implications are needed.
Therefore, the current trend of niosome preparation is to increase the efficiency of controlled drug release, avoid the use of organic solvents, and reduce the particle size in just one step; multiple methods, such as the ball milling method222 and bubble method14, have recently been studied. Another development goal of niosomes is to optimize their preparation technique, aiming for improved quality and scalability to produce a greater number of niosomes. Furthermore, the future goal of niosomes is not only to encapsulate the active ingredient into vesicles but also to load the targeted drug at the site of action, such as the central nervous system, to significantly increase permeability, cross the blood‒brain barrier and increase bioavailability via modification with ligands226. Niosomes decorated with ligands have been shown to prolong their presence in blood circulation227 and enhance their ability to traverse the blood‒brain barrier19.
Although there are reports of niosomes loaded with antifungal, antineoplastic, and antidiabetic compounds for intravenous, oral, and transdermal administration, compared with liposomes, most studies are still and 228. The main application of niosomes is in transdermal drug delivery systems, which are mainly topical products, such as Lancôme's229, 15. Since then, no drug-based niosomes have been marketed orally or injected. The limitation of the transdermal route for the treatment of certain diseases, such as cancer or infection, is extending the duration of treatment and leading to poor patient compliance. Because niosomes provide a large area and many ligand binding sites, such as antibodies, peptide chains, and folic acid230, they can be modified to diversify drug delivery pathways and increase the application efficiency of niosomes. In addition to loading pharmaceutical compounds, the use of natural therapeutic molecules (e.g., curcumin231 and morusin232) is also a focus of research because of their ability to encapsulate both lipophilic and hydrophilic molecules owing to the presence of amphiphilic surfactants, which help absorb natural-origin molecules into the body, reduce the side effects of some therapeutic drugs and synergistically increase the therapeutic potential of drugs.
Conclusion
Niosomes have long been known as effective drug delivery vehicles. The characteristics of niosomes can be altered by adjusting their size and structure during the preparation process. Niosomes may offer additional benefits in terms of stability and cost compared with liposomes. Additionally, the application of niosomes via oral, dermal, ocular, and respiratory routes is viable for safe long-term treatment and focused medication delivery. Although topical niosomes appear to be a feasible and promising strategy for therapeutic and cosmetic applications, further investigation is needed to understand the mode of interaction between niosomal particles and stratum corneum lipids and their impact on drug penetration. More clinical investigations and postmarketing surveillance should be conducted to obtain more evidence for the safety and efficacy of niosomes in humans. Although controlled clinical studies are needed to acquire additional information about niosome safety for long-term treatment, stability, and bioavailability effects, niosomes are attractive candidates for treating ocular diseases. Vaccine and biotechnology product delivery are also potential areas of research that should attract more attention. The encouraging results from niosomal systems and the emerging discoveries in this field will likely lead to more viable commercial niosomal products.
List of abbreviations
CS Chitosan
EE% Entrapment efficiency
FLP Finasteride-loaded proniosomes
HLB Hydrophilic‒lipophilic balance
LUVs Large unilamellar vesicles
LXN Lomefloxacin HCl
MLVs Multilamellar vesicles
MTX Methotrexate
PDI Polydispersity index
PEG Polyethylene glycol
R6G Rhodamine 6G
SC Stratum corneum
SUVs Small unilamellar vesicles
Competing interests
The authors declare that they have no competing interests.
Acknowledgments
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under grant number DN2022-44-01.
Authors’ contributions
The concepts were proposed by Minh Hien Nguyen and Minh Tri Le. Minh Hien Nguyen, Thien Han Nguyen Le, Tran Phuoc Thuan Nguyen, Thi Ngoc Tam Le, Thi Yen Nhi Nguyen, Kim Anh Nguyen, and Thi Tan Pham undertook to collect, summarize, and evaluate researches, news. All authors contributed to drafting and revising the final manuscript.

