Effects of CoCl2-Induced Hypoxia on HepG2 Cells: Increased VEGF and GLUT1 Gene Expression and Enhanced Doxorubicin Resistance
- VNUHCM-US Stem Cell Institute, University of Sciences, Vietnam National University Ho Chi Minh City, Viet Nam
Abstract
Introduction: This study investigates the effects of cobalt chloride (CoCl₂), a hypoxia-mimicking agent, on HepG2 liver cancer cells, focusing on cell survival, hypoxia-related gene expression, chemotherapy sensitivity, and drug efflux capacity. Hypoxia is a critical feature of tumor microenvironments linked to cancer progression and therapy resistance, and CoCl₂ is widely used to simulate these conditions in vitro. The aim was to evaluate how varying concentrations and exposure times of CoCl₂ influence cellular responses, providing insights into hypoxia-driven mechanisms in liver cancer.
Methods: HepG2 cells were treated with CoCl₂ at concentrations of 50, 100, 150, 200, and 250 µM over 12, 24, and 48 hours. Cell viability was assessed to determine cytotoxicity. Gene expression levels of hypoxia-associated markers—vascular endothelial growth factor (VEGF), glucose transporter 1 (GLUT1), and hypoxia-inducible factor 1-alpha (HIF-1α)—were quantified at 72 and 96 hours. Chemotherapy sensitivity was evaluated using doxorubicin, with IC₅₀ values calculated to measure resistance. Drug efflux capacity was analyzed via rhodamine 123 retention assays.
Results: Lower CoCl₂ concentrations (50–100 µM) showed no significant cytotoxicity, while higher doses (150–250 µM) reduced cell viability in a concentration- and time-dependent manner. CoCl₂ induced marked upregulation of VEGF and GLUT1 at 72 and 96 hours, despite stable HIF-1α levels, suggesting enhanced angiogenesis and metabolic adaptation. Treated cells exhibited reduced sensitivity to doxorubicin, with increased IC₅₀ values, and a twofold rise in the rhodamine 123-effluxing subpopulation, indicating enhanced drug resistance and efflux capacity.
Conclusion: CoCl₂ effectively mimics hypoxia in HepG2 cells, driving adaptive responses such as angiogenesis, metabolic shifts, and drug resistance. While valuable for studying hypoxia-related pathways, optimal experimental outcomes require careful selection of CoCl₂ concentration and exposure duration. These findings highlight the utility of CoCl₂ in modeling tumor microenvironment challenges and underscore the need to standardize protocols for translational relevance.
Introduction
Hypoxia, a condition characterized by reduced oxygen availability, is a common feature of the tumor microenvironment and has been implicated in the development of resistance to chemotherapy in various cancers, including hepatocellular carcinoma (HCC). The human liver cancer cell line HepG2, which is often used as a model for HCC, is highly resistant to chemotherapeutic agents such as doxorubicin under hypoxic conditions. This resistance is a major obstacle in the effective treatment of HCC, necessitating a deeper understanding of the underlying mechanisms.
Recent studies have highlighted the role of hypoxia-inducible factor (HIF) and ATP-binding cassette (ABC) transporters in mediating drug resistance. For example, HIF-1α, a key transcription factor activated under hypoxic conditions, has been shown to upregulate the expression of ABC transporters such as P-glycoprotein (Pgp) and breast cancer resistance protein (BCRP), which actively efflux chemotherapeutic drugs out of cancer cells, thereby reducing their efficacy 1, 2. Additionally, nuclear factor erythroid 2-related factor 2 (NRF2) and poly (ADP‒ribose) polymerase 1 (PARP1) have been implicated in the increased efflux of doxorubicin and the repair of doxorubicin-induced DNA damage, respectively, further contributing to drug resistance in hypoxic HepG2 cells 3
The interplay between these molecular pathways under hypoxic conditions suggests a complex network of regulatory mechanisms that promote chemoresistance. For example, the combination of doxorubicin-induced reactive oxygen species (ROS) production and HIF-1α activity has been shown to synergistically induce the expression of Pgp and BCRP, thereby increasing drug resistance 1. Moreover, gene therapy approaches targeting HIF-1α have demonstrated potential in enhancing the therapeutic efficacy of doxorubicin by downregulating HIF-1α and vascular endothelial growth factor (VEGF) expression, leading to reduced tumor growth and increased apoptosis in HepG2 cells 3.
Cobalt chloride (CoCl), which is a hallmark of the tumor microenvironment, is widely used in cancer research as a hypoxia mimetic agent to simulate low-oxygen conditions. Hypoxia in tumors is associated with aggressive growth, therapy resistance, and the maintenance of cancer stem cell properties. CoCl induces hypoxia by stabilizing HIF-1α, which then translocates to the nucleus and activates the transcription of various genes involved in angiogenesis, metabolism, and survival. This process is crucial for studying the hypoxic response in cancer cells, as it allows researchers to investigate the molecular and cellular adaptations to low-oxygen conditions without the need for specialized hypoxia chambers 4. CoCl has been used to create in vitro models of cancer cell dormancy. For example, it has been shown to mimic the hypoxic regulation of dormancy in breast cancer cell lines (MCF-7 and MDA-MB-231) and ovarian cancer cells (OVCAR-3), highlighting its utility in studying the mechanisms underlying cancer cell dormancy and reactivation. CoCl-induced hypoxia upregulates vascular endothelial growth factor (VEGF) and cyclooxygenase-2 (COX-2), which are critical for angiogenesis and metastasis. This has been demonstrated in prostate cancer cell lines, where CoCl treatment significantly increased VEGF expression and secretion, particularly in highly metastatic cells 5. CoCl has also been employed in in vivo models, such as for the transplantation of tumor xenografts into fertilized chicken eggs. This method successfully induces hypoxia in pancreatic ductal adenocarcinoma (PDA) cells, increasing the expression of hypoxia markers and cancer stem cell features without adverse effects on the host 6. In murine mammary cancer cells, CoCl treatment resulted in increased tumorigenicity and changes in the expression of hypoxia markers 7. In lung adenocarcinoma cells, CoCl treatment increases the population of cancer stem-like cells, which exhibit increased resistance to chemotherapy 8. The role of CoCl in modulating the expression of adhesion molecules such as ICAM-1 and VCAM-1 has been investigated in colorectal cancer cells. These molecules are important for cancer progression and resistance to treatment, and their expression can be influenced by the hypoxic conditions induced by CoCl9.
In the HepG2 cell line, CoCl has been shown to reduce apoptosis induced by agents such as tert-butyl hydroperoxide (t-BHP) and serum deprivation, suggesting that HIF-1α may play an antiapoptotic role under the hypoxic conditions induced by CoCl10. However, CoCl has also been reported to inhibit the proliferation of HepG2 cells and stimulate apoptosis by increasing caspase-3 activity at relatively high concentrations 11. These findings indicate that the effects of CoCl are concentration-dependent and condition specific, necessitating further research to optimize and clarify these effects. Moreover, studies using CoCl to induce hypoxia are still limited, and experimental conditions such as concentration and exposure time need to be optimized to ensure accurate and reproducible results. Understanding the mechanisms of HIF-1α and related signaling pathways is also crucial, as this information could provide valuable insights for therapies targeting liver cancer and hypoxia-related conditions. Therefore, further investigation into the effects of CoCl on HepG2 cells is essential and could lead to new therapeutic approaches.
In this study, we aimed to investigate the effects of cobalt chloride (CoCl)-induced hypoxia on gene expression and doxorubicin resistance in HepG2 cells. By elucidating the molecular mechanisms underlying hypoxia-induced chemoresistance, we hope to identify potential therapeutic targets to overcome drug resistance and improve the efficacy of doxorubicin in the treatment of HCC.
Materials and Methods
Cell Culture
HepG2 cells were obtained from the ATCC cell bank and cultured in DMEM (Dulbecco's modified Eagle’s medium, USA) supplemented with 10% fetal bovine serum (Thermo Fisher Scientific, Waltham, MA) and 1% penicillin‒streptomycin (HyClone, USA). The cells were maintained in a humidified incubator at 37°C with 5% CO2.
Establishing the hypoxia model
HepG2 cells were cultured in a T25 flask until they reached a confluency of 70–80%, ensuring optimal cell growth conditions. Next, the HepG2 cells were carefully plated at a density of 2,000 cells per well in 96-well plates along with the appropriate medium to support their growth and viability. The cells were subsequently subjected to CoCl (TMMedia, India) after a designated 24-hour incubation period to induce hypoxic conditions. The cells were exposed to varying concentrations of CoCl (0, 50, 100, 150, 200, and 250 μM) for different durations ranging from 24 to 72 hours to perform the resazurin staining assay. Each experimental condition was replicated three times.
Analysis of HepG2 Cell Subpopulations under Hypoxic Conditions via Flow Cytometry
The cells were maintained in T25 flasks under normoxic conditions and hypoxic conditions induced by 100 μM CoCl in a cell culture incubator with 5% CO at 37°C for 72 hours. After 72 hours, the cells were detached with 1 mL of detachment solution (Regenmed Lab, Vietnam). The detached cells were transferred to 15 mL centrifuge tubes and centrifuged at 300 × g for 5 minutes. The supernatant was discarded, and the cell pellets were collected. This process was performed under both normoxic and hypoxic conditions. The cells were resuspended in 1 mL of PBS and then split evenly into two separate 15 mL centrifuge tubes for the unstained and sample groups under both normoxic and hypoxic conditions. Sample tubes were stained with Rhodamine 123 (R1008, Sigma, USA) at a final concentration of 0.1 μg/mL. The tubes were incubated in the dark at 37°C with 5% CO for 30 minutes. After staining, the cells were washed twice with 1 mL of PBS. The cells were resuspended in 1 mL of PBS. Flow cytometry was performed via a flow cytometer equipped with appropriate filters for Rhodamine 123 fluorescence detection. The data were collected and analyzed to assess the subpopulations of HepG2 cells under normoxic and hypoxic conditions. Statistical analysis was performed via GraphPad Prism software. The results are expressed as the mean ± standard deviation (SD) of n=3 samples. Statistical significance was determined via a two-tailed unpaired t test, with a p value < 0.05 considered significant.
Quantitative PCR analysis to assess the mRNA levels of , and
RT‒qPCR was conducted to quantitatively assess alterations in the expression levels of HIF-1α, VEGF, and GLUT1 mRNAs in HepG2 cells following treatment with varying concentrations of 0, 50, and 100 μM CoCl for 72 and 96 hours. HepG2 cells, sourced from a range of conditions, were cultivated in six-well plates to facilitate the experimental procedures. The extraction of total RNA from the cells was carried out via an easy-BLUETM kit (iNtRON Biotechnology, Korea). Subsequent quantitative PCR analyses of HIF-1α, VEGF, and GLUT1 mRNA levels were performed via the Luna® Universal One-Step RT‒qPCR Kit (New England Biolabs Inc., US) following the instructions provided by the manufacturer. The specific sequences of primers used for quantitative PCR were as follows: HIF1α - forward: 5’-TGCTTGCCAAAAGAGGTGGA-3’, reverse: 5’-GGGGCCAGCAAAGTTAAAGC-3’; VEGF - forward: 5'-GGACATTGCTGTGCTTTGGG-3’, reverse: 5’-ATGGGCTGCTTCTTCCAACA-3’; GLUT1 - forward: 5’-CTGTCGTGTCGCTGTTTGTG-3’, reverse: 5’-AAAGATGGCCACGATGCTCA-3’; and β-actin - forward: 5’-GACTTAGTTGCGTTACACCCTTTCT-3’, reverse: 5’-GAACGGTGAAGGTGACAGCAGT-3’. All primers were designed via the Primerblast webtool available at https://www.ncbi.nlm.nih.gov/tools/primer-blast/. The thermocycling conditions involved initial denaturation at 95°C for 1 minute, followed by annealing at 55°C for 10 minutes, and then 40 cycles at 95°C for 10 seconds and 60°C for 30 seconds, specifically for HIF-1α, VEGF, and GLUT1. The relative mRNA levels of HIF-1α, VEGF, and GLUT1 were normalized to those of β-actin. The entire experimental procedure was performed in triplicate.
Doxorubicin treatment
HepG2 cells were seeded into 96-well plates at a density of 2000 cells per well in 100 μL of culture medium. The plates were incubated in a cell culture incubator at 37°C with 5% CO for 24 hours to allow for cell adhesion. After 24 hours, cell adhesion was checked under a microscope and recorded prior to doxorubicin treatment. A stock solution of doxorubicin (Ebewe Arzneimittel, Austria) was diluted in culture medium to a final concentration of 2 μM. The cells were treated with doxorubicin at concentrations of 2000, 1000, 500, 250, 125.5, and 62.5 nM. The control sample was supplemented with PBS.
Alamar Blue Assay
After 24 hours of doxorubicin treatment, the culture medium was retained, and 10 μL of Alamar blue reagent (DAL1025, Invitrogen, US) was added to each well at a final concentration of 10 µg/ml. The cells were incubated in the dark at 37°C with 5% CO for 45 minutes. The fluorescence intensity was measured using a DTX880 plate reader at an excitation wavelength of 570 nm.
Data analysis
The fluorescence data were analyzed via GraphPad Prism software to determine the effect of the half-maximal inhibitory concentration (IC) of doxorubicin on HepG2 cell proliferation. The IC value was calculated by plotting the fluorescence intensity against the doxorubicin concentration and fitting the data to a sigmoidal dose‒response curve. Statistical analyses were conducted to compare the treated and control groups.
Statistical
The data are expressed as the means ± standard deviations (SDs) of three independent experiments. Statistical significance was determined via one-way ANOVA followed by Tukey's post hoc test, with a p value < 0.05 considered significant.
RESULT
CoCl concentrations of 50 and 100 µM had no significant effect on the survival of HepG2 cells
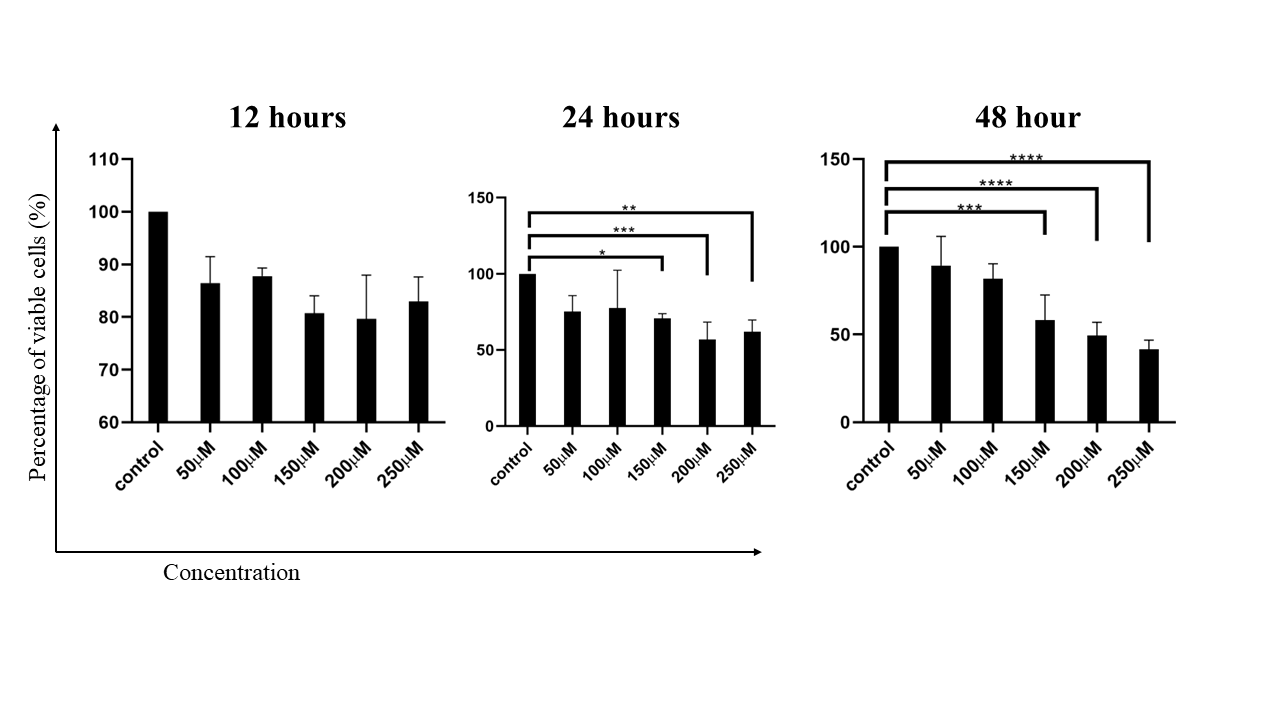
HepG2cell viability under CoCl2 treatment. The bar graph shows the viability of HepG2 cells treated with different concentrations of CoCl2 (50, 100, 150, 200, and 250 µM) at 12, 24, and 48 hours. Cell viability was assessed via a Reasurin dye assay, with the results indicating no significant impact at 50 and 100 µM, whereas higher concentrations (150, 200, and 250 µM) significantly reduced cell viability. The error bars represent the standard deviation (SD), with statistical significance indicated by asterisks (*p < 0.05, **p < 0.01).
When the concentration of CoCl applied to the HepG2 cell line at concentrations of 50, 100, 150, 200, and 250 µM for 12 hours, 24 hours, and 48 hours, the results revealed that at concentrations of 50 and 100 µM at the time of the survey, there was no statistically significant difference in cell survival among the control groups. These findings indicate that at lower concentrations of CoCl (50 and 100 µM), there was no significant difference in the survival of HepG2 cells compared with that of the control group. These findings suggest that these concentrations are not sufficient to induce notable cytotoxic effects or apoptosis in the HepG2 cell line within the given time frames. This observation aligns with the results of several studies showing that CoCl can reduce apoptotic cell death in HepG2 cells under certain conditions, suggesting that lower concentrations might not be sufficient to trigger significant apoptosis 10. CoCl significantly inhibited the proliferation of HepG2 cells and induced apoptosis at relatively high concentrations, but the effects at relatively low concentrations were not highlighted, implying a threshold effect 12. A previous study revealed a concentration-dependent increase in apoptosis rates with CoCltreatment, with significant effects observed at higher concentrations (200 µM and above), suggesting that 50 and 100 µM CoCl may not have an impact 13.
While the specific results for higher concentrations (150, 200, 250 µM) were not detailed in the provided data, it is reasonable to infer from the literature that higher concentrations (150, 200, 250 µM) would likely result in increased cytotoxicity. This result demonstrated a clear concentration-dependent increase in apoptosis rates with CoCl treatment, with significant effects observed at concentrations of 200 µM and above 13. CoCl inhibited cell growth in a concentration-dependent manner, suggesting that higher concentrations had a more pronounced effect on cell viability 14. The time dependency of the effects of CoCl on HepG2 cells is also crucial. Studies suggest that prolonged exposure to CoCl increases its cytotoxic effects 10. The results indicate that, compared with the control, CoCl at concentrations of 50 and 100 µM does not significantly affect HepG2 cell survival. However, higher concentrations (150, 200, and 250 µM) are likely to induce significant cytotoxic effects and apoptosis, as supported by the literature. The time of exposure also plays a critical role, with longer durations leading to more pronounced effects. However, further studies are needed to elucidate the exact mechanisms and thresholds for CoCl-induced cytotoxicity in HepG2 cells.
The use of CoCl as a hypoxia-inducing agent has been extensively studied, with varying results. Zhang (2014) reported that CoCl pretreatment did not increase hypoxia tolerance in mice 15, whereas Zhigalova (2015) reported that it failed to induce alterations in the glycolysis/gluconeogenesis pathway in renal cancer cells 16. Shweta (2014) further highlighted the influence of CoCl on the inflammatory response, particularly through the NF-κB signaling pathway 17. However, Jin (2012) reported a radioprotective effect of low-dose CoCl on HepG2 cells, suggesting a potential role in inducing HIF-1α expression and clearing reactive oxygen species 18. These findings collectively suggest that while CoCl may not significantly affect the proliferation of HepG2 cells at certain concentrations, it can still be used as a hypoxia mimic inducer, particularly at low doses.
The impact of CoCl on the growth rate of HepG2 cells
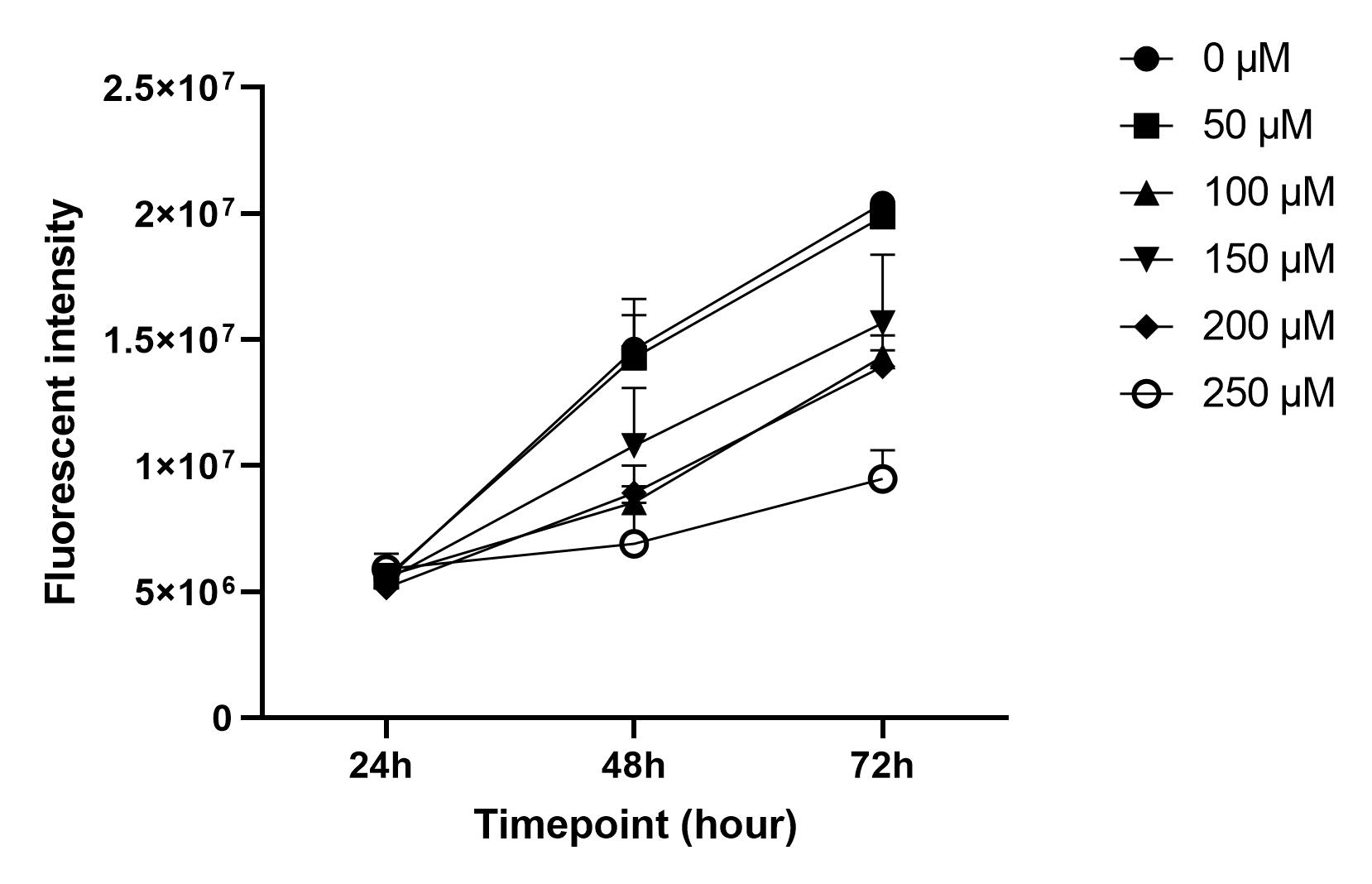
Effects of CoCl2 on the cell growth rate over time.The growth rate curves of HepG2 cells treated with different concentrations of CoCl2 (0 µM, 50 µM, 100 µM, 150 µM, 200 µM, and 250 µM) were evaluated at 24, 48, and 72 hours. The error bars represent the means ± SDs (n = 3).
The results of the present study indicate that the cell growth rate curves for CoCl concentrations of 50 µM and 100 µM do not differ significantly from those of the control group at 24, 48, and 72 hours (Figure 2). The CoCl-induced hypoxic cell model of oral squamous cell carcinoma was used to investigate the effects of CoCl on cell behavior. Higher concentrations of CoCl (150 µM and above) significantly affect cell proliferation, apoptosis, and migration 19. However, at lower concentrations (50 µM and 100 µM), the impact on the cell growth rate was minimal, which aligns with the findings of the present study. The results suggest that CoCl concentrations of 50 µM and 100 µM do not significantly alter the cell growth rate compared with that of the control, which is consistent with findings from related studies on cell growth under controlled conditions and varying external factors.
CoCl-induced upregulation of VEGF and GLUT1 in HepG2 cells
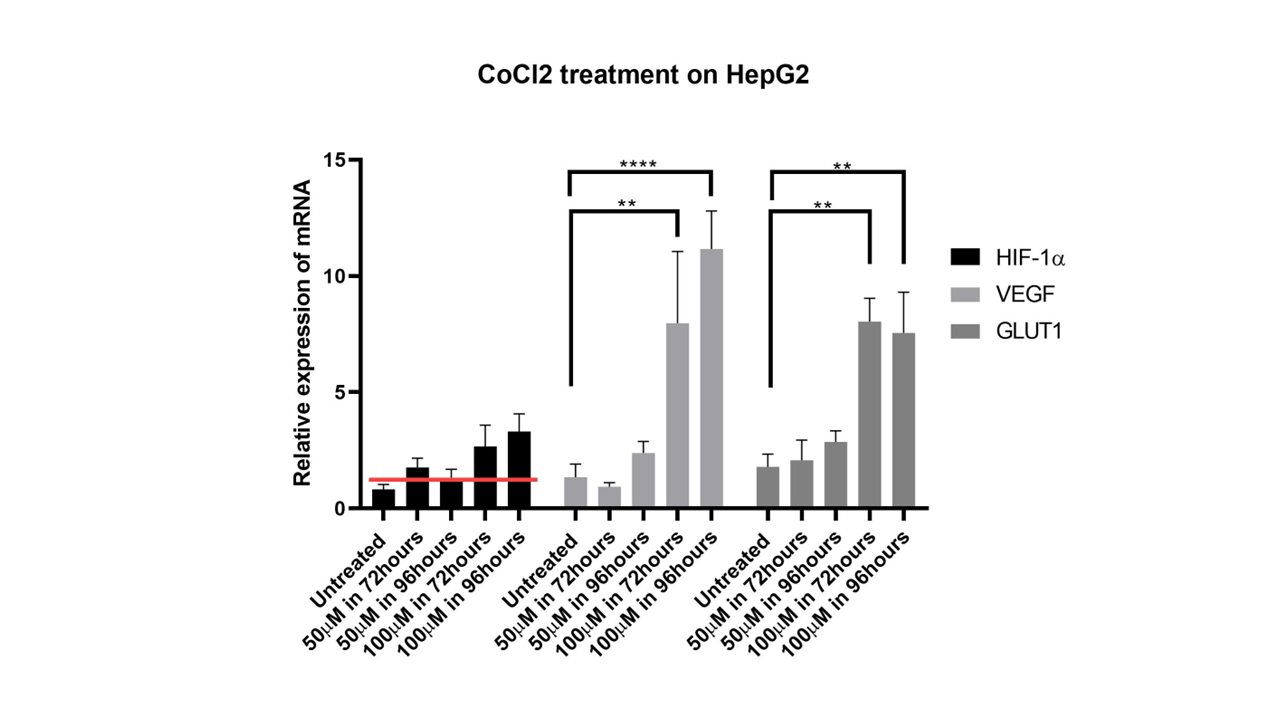
Geneexpression levels in HepG2 cells treated with CoCl2. The graphs depict the relative expression levels of VEGF, GLUT1, and HIF-1α in HepG2 cells treated with 100 µM CoCl2 at 72 and 96 hours. VEGF and GLUT1 levels significantly increased at both time points compared with those of the control, whereas HIF-1α levels remained elevated but did not significantly differ from those of the control. The expression levels are normalized to control values, with error bars indicating the standard error of the mean (SEM).
The results indicated that treatment with CoCl significantly increased the expression of VEGF and GLUT1 at 72 and 96 hours, while HIF-1alpha expression did not significantly differ from that of the control. However, HIF-1alpha expression was still higher than that in the control at these time points. This finding can be discussed in the context of literature on the role of HIF-1alpha, VEGF, and GLUT1 in hypoxic conditions and their regulation.
Hypoxia-inducible factor-1alpha (HIF-1alpha) is a key regulator of the cellular response to hypoxia, primarily by activating the transcription of genes involved in angiogenesis, such as vascular endothelial growth factor (VEGF) and glucose transporter 1 (GLUT1). The finding that CoCl treatment significantly increases VEGF and GLUT1 expression aligns with the established role of HIF-1alpha in promoting angiogenesis and metabolic adaptation under hypoxic conditions.
The significant increase in VEGF and GLUT1 expression upon CoCl treatment is consistent with findings from multiple studies. For example, HIF-1alpha has been shown to regulate VEGF expression, which is crucial for angiogenesis in various cancer models, including gliomas and ovarian cancer 20, 21. Similarly, GLUT1, a key glucose transporter, is upregulated under hypoxic conditions to facilitate increased glucose uptake, supporting cellular metabolism in low-oxygen environments 21. Interestingly, while HIF-1alpha expression did not significantly differ from that of the control, it was still greater at 72 and 96 hours. These findings suggest that while HIF-1alpha levels may not drastically change, its activity or stability might be enhanced under CoCl treatment, leading to increased transcription of its target genes, VEGF and GLUT1. These findings are supported by studies showing that HIF-1alpha can be stabilized and activated under hypoxic conditions or by hypoxia-mimicking agents such as CoCl, leading to increased VEGF secretion and tumor growth 22. The elevated expression of VEGF and GLUT1 under CoCl treatment has significant implications for tumor growth and angiogenesis. VEGF is a potent angiogenic factor that promotes the formation of new blood vessels, which are essential for tumor growth and metastasis 20, 23. The upregulation of GLUT1 ensures an adequate supply of glucose to meet the metabolic demands of rapidly proliferating tumor cells under hypoxic conditions 21. Therefore, the observed increase in VEGF and GLUT1 expression suggests that CoCl treatment could increase angiogenesis and metabolic adaptation, potentially contributing to tumor progression. CoCl treatment significantly increased VEGF and GLUT1 expression, whereas HIF-1alpha expression remained relatively unchanged but was elevated, highlighting the complex regulation of hypoxia-responsive genes. These findings are consistent with the role of HIF-1alpha in mediating the cellular response to hypoxia, promoting angiogenesis, and metabolic adaptation through the upregulation of VEGF and GLUT1. These findings underscore the potential therapeutic implications of targeting HIF-1alpha and its downstream pathways in cancer treatment.
Impact of CoCl-Induced Hypoxia-Mimicking Conditions on Doxorubicin Sensitivity in HepG2 Cells
Sensitivity of HepG2 Cells to Doxorubicinunder CoCl2 Treatment. The IC50 values of doxorubicin in HepG2 cells under normal conditions and following treatment with 100 µM CoCl2. The IC50 value was significantly greater in CoCl2-treated cells than in control cells, indicating reduced sensitivity to the chemical drug. The error bars denote the SDs, with statistical significance marked by asterisks (***p < 0.001).
The IC of doxorubicin in HepG2 cells significantly differed under normal conditions and when the cells were treated with 100 µM CoCl, with values of 286.16±19.97 and 505.66±48.64 nM, respectively, as revealed by 2-way ANOVA12. This disparity suggests that the presence of CoCl impacts the sensitivity of HepG2 cells to doxorubicin. CoCl, a hypoxia-mimicking agent, may alter the cellular response to chemotherapy, potentially affecting the efficacy of doxorubicin in cancer treatment. The higher IC value observed in cells treated with CoCl indicates a reduced sensitivity to doxorubicin, highlighting the importance of understanding how hypoxia-mimicking conditions can influence the effectiveness of chemotherapy in liver cancer cells such as HepG2 cells.
CoCl-induced hypoxia-mimicking conditions have been shown to contribute to doxorubicin resistance in HepG2 cells through various mechanisms. CoCl induces AGR2, which enhances HIF-1α expression. This upregulation increases MDR1 levels, thereby limiting doxorubicin intake and leading to doxorubicin resistance 24. NRF2/ABCB1-mediated efflux and PARP1-mediated DNA repair have been identified as key factors in this resistance 3. Additionally, the combination of doxorubicin and CoCl has been shown to promote the expression of Pgp and BCRP, both of which are associated with drug resistance, in colon cancer cells under hypoxic conditions 1. Finally, CoCl-induced HIF-1alpha overexpression has been linked to cellular resistance to photodynamic therapy, suggesting a potential role in doxorubicin resistance as well 25.
Enhanced Efflux of Rhodamine 123 in HepG2 Cell Subpopulations Following 72-Hour CoCl Treatment
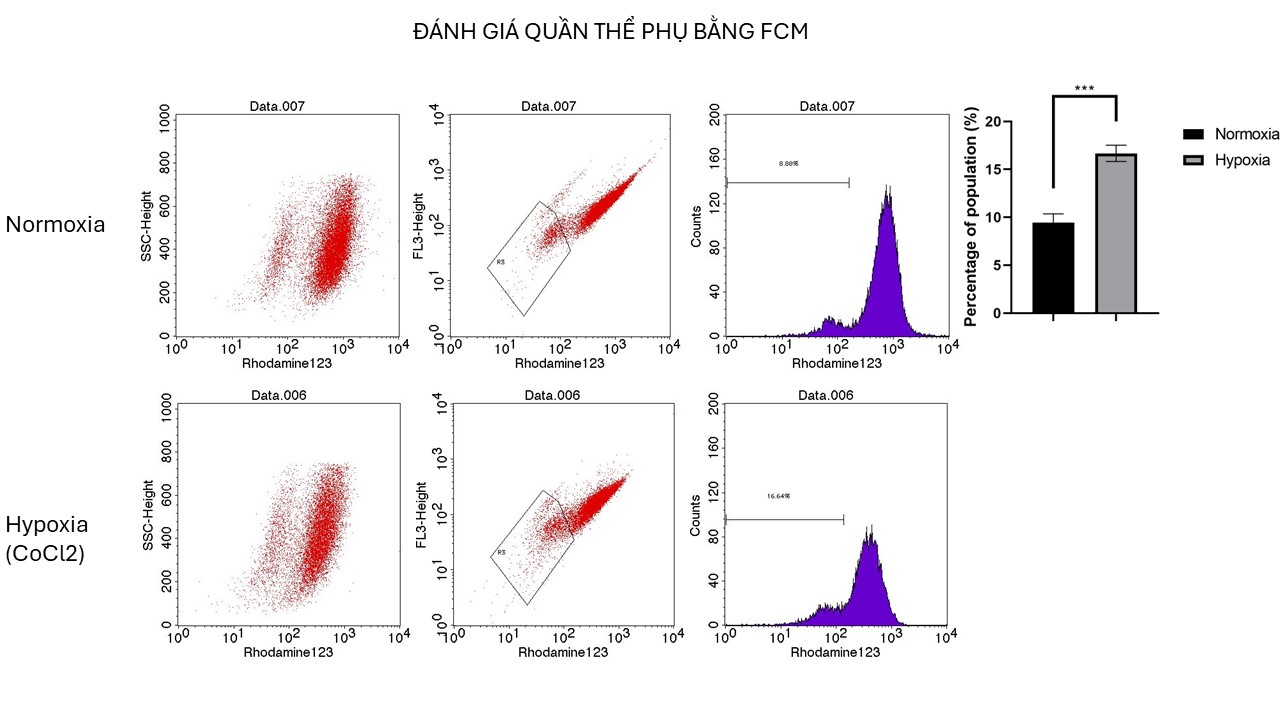
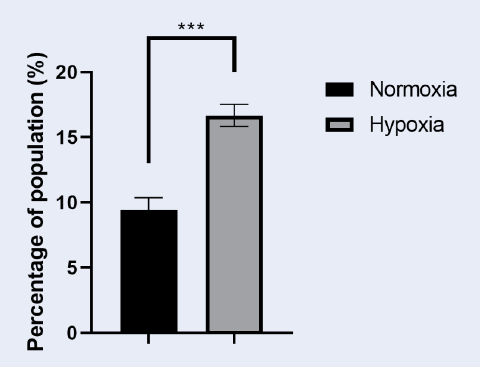
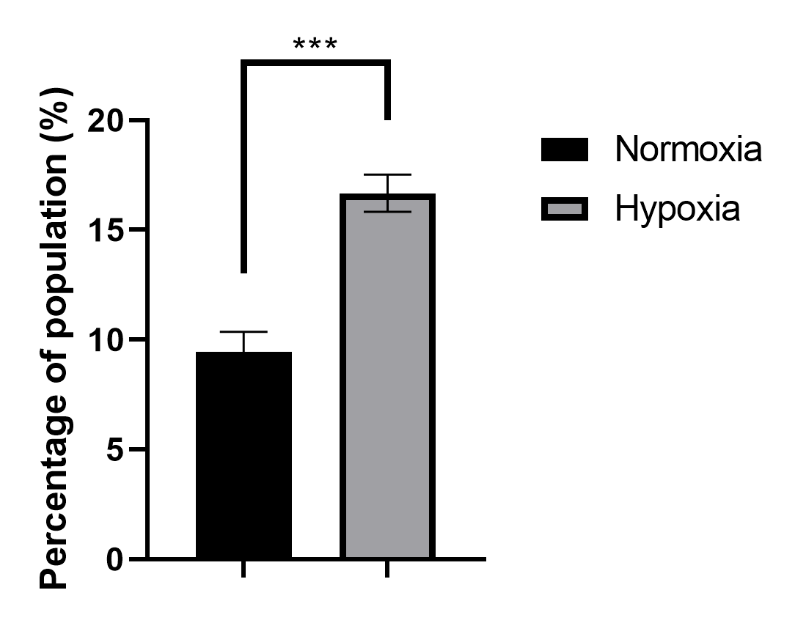
Enhanced Efflux Capacity of HepG2 Cellsafter CoCl2 Treatment. Histogram comparing the percentage of HepG2 cells capable of effluxing rhodamine 123 after 72 hours of treatment with 100 µM CoCl2 with that of untreated cells. The treated group showed a significant increase in the subpopulation of efflux-capable cells, indicating enhanced drug efflux capacity. The error bars represent the SDs, with significant differences indicated by asterisks (**p < 0.01).
Rhodamine 123 (Rho123) is a fluorescent dye widely utilized in biological research to investigate mitochondrial function and drug resistance mechanisms. In the context of HepG2 cells, a human liver cancer cell line, understanding the interaction between Rho123 and drug resistance can illuminate the mechanisms of multidrug resistance (MDR) and suggest potential therapeutic strategies. Compared with drug-sensitive cells, drug-resistant cells exhibit significantly lower accumulation of Rho123, indicating active efflux of Rho123 from resistant cells 26. Consequently, the cytotoxicity of Rho123 is markedly greater in drug-sensitive cells, with ICvalues up to 1000 times greater in resistant cells 26. This is attributed to Rho123 being a substrate for P-glycoprotein (P-gp), a crucial MDR player that actively pumps Rho123 out of resistant cells, reducing its intracellular concentration and cytotoxic effects 27. Direct binding of Rho123 to P-gp has been confirmed, emphasizing its role in the efflux mechanism contributing to drug resistance. The P-gp inhibitor verapamil significantly increases Rho123 accumulation in resistant cells, lowering the IC value by more than 1000-fold and suggesting that P-gp inhibition can reverse Rho123 resistance 26. Additionally, Rho123 impacts mitochondrial function differently in drug-sensitive and drug-resistant cells, causing a greater increase in respiratory State 4 and slightly greater inhibition of State 3 in resistant cells, although these effects do not entirely account for the differential drug sensitivity 28.
The study evaluated the impact of CoCl treatment on a subpopulation of HepG2 cells capable of pumping out Rhodamine123. The results indicated that after 72 hours of CoCl treatment, the subpopulation of CoCl-treated cells doubled compared with that of untreated cells, with values of 16.68±0.85 for CoCl-treated cells and 9.45±0.89 for untreated cells. This increase in the subpopulation suggests that CoCl treatment may increase the efflux capacity of cells, potentially affecting their ability to remove rhodamine123. These findings highlight the potential role of CoCl in modulating the subpopulation of cells involved in Rhodamine123 transport, indicating a significant impact on cellular processes related to dye efflux mechanisms.
DISCUSSION
The effects of different concentrations of CoCl on HepG2 cells were assessed at various time points 12. The results indicated that at concentrations of 50 and 100 μM, there was no significant difference in cell survival compared with that of the control group at the time of evaluation 34. These findings suggest that lower doses of CoCl, specifically 50 and 100 μM, did not significantly affect cell viability within the observed time frame. However, further analysis at additional time points and concentrations is necessary to fully understand the dose-dependent effects of CoCl on HepG2 cells 5. These findings highlight the complex relationship between CoCl concentrations and their impact on cell survival, emphasizing the need for comprehensive investigations into the cellular responses to different doses of CoCl.
In our study, we found that the expression of the HIF-alpha gene in batches of cells treated with CoCl was greater than that in batches of untreated HepG2 cells treated with 100 µM CoCl at the 72- and 96-hour time points. We hypothesize that CoCl induces hypoxia and stabilizes HIF-1, which can modulate apoptosis and affect the MMP. Therefore, it is not necessary for HIF gene expression to be significantly increased; merely stabilizing the HIF protein can induce the apoptotic effect. This hypothesis aligns with findings from various studies. CoCl exposure can indeed induce hypoxia-like conditions, leading to the stabilization of HIF-1α 29. Stabilized HIF-1α has been shown to play a role in modulating apoptosis by regulating the expression of apoptotic biomarkers such as Bax and Bcl-2 30. Additionally, HIF-1α has been found to reduce the degree of apoptosis induced by oxidative stress by influencing the expression of antiapoptotic proteins such as Bcl-2 and Bcl-XL 31. These data suggest that stabilizing HIF-1α can indeed impact apoptosis and other cellular processes without significantly increasing HIF gene expression 22.
We found a statistically significant increase in GLUT1 and VEGF gene expression in HepG2 cells treated with CoCl, indicating a potential association with hypoxia-like responses and cancer progression 32, 33. CoCl-induced hypoxic conditions have been shown to upregulate GLUT1 expression, facilitating glucose uptake in cancer cells, which may promote tumorigenesis and metastasis 34, 35. Additionally, elevated VEGF gene expression suggests a potential role in promoting angiogenesis, which is crucial for tumor growth and metastasis 36. These findings highlight the impact of CoCl-induced hypoxia on molecular responses, potentially influencing cellular behavior, such as increased oxidative stress and apoptotic events. The upregulation of the GLUT1 and VEGF genes under hypoxic conditions may contribute to the aggressive nature of HepG2 cancer cells, emphasizing the importance of understanding these molecular changes in cancer progression.
Subpopulations of cells treated with CoCl exhibit differences in the efflux of Rhodamine123. CoCl treatment influences the efflux of Rhodamine123 in cells, impacting their multidrug resistance characteristics. Studies have shown that CoCl-treated cells exhibit altered efflux patterns of rhodamine 123, potentially affecting drug sensitivity 37. Additionally, the presence of P-glycoprotein (Pgp), a product of the multidrug resistance gene, in cells plays a role in the efflux of Rhodamine123 38. Furthermore, the intracellular binding of drugs, including Rhodamine123, is affected by the activity of ABC transporters such as MRP1, influencing drug resistance mechanisms 39. These findings suggest that CoCl treatment can modulate the efflux of Rhodamine123 through interactions with Pgp and ABC transporters, impacting the cellular response to multidrug resistance. In fact, in this study, we found that the HepG2 cell batch treated with CoCl had an IC for doxorubicin of 505.66±48.64 at 48 hours, which was significantly greater than the IC for doxorubicin in the HepG2 cell batch not cotreated with CoCl. These findings indicate that doxorubicin resistance occurs in HepG2 cells treated with CoCl. Our hypothesis is that CoCl induces hypoxia and stabilizes HIF-1, which can modulate apoptosis and affect the mitochondrial membrane potential (MMP). This stabilization likely influences the function of multidrug resistance (MDR) proteins, such as Pgp and ABC transporters. The increased IC of doxorubicin in CoCl-treated HepG2 cells suggests that hypoxia-mimicking conditions induced by CoCl increase the efflux capability of these transporters, reducing the intracellular concentration of doxorubicin and thereby increasing drug resistance. Our findings are consistent with other studies that have shown a relationship between hypoxia-induced HIF-1 stabilization and increased expression or activity of MDR proteins. For example, research has demonstrated that hypoxic conditions lead to the upregulation of Pgp, resulting in increased drug efflux and resistance 40, 41. Similarly, the stabilization of HIF-1 under hypoxia can alter cellular responses to chemotherapy by modulating the activity of ABC transporters 42, 43.
CoCl treatment influences Rhodamine123 efflux in HepG2 cells by impacting the mitochondrial membrane potential (MMP) and cellular transporters. CoCl induces hypoxia and stabilizes HIF-1, which can modulate apoptosis and affect the MMP 44. Additionally, CoCltreatment decreases GPC3 expression via the HIF-1α/c-myc axis, impacting cell growth 10. Furthermore, exposure to CoCl leads to oxidative stress and inflammation 12. Rhodamine 123, a mitochondrial probe, is transported by organic cation transporters (OCTs), including hOCT1 and hOCT2 45. Dye uptake is carrier-mediated and can inhibit hOCT1 and hOCT2 activities, affecting the efflux of substrates such as tetraethylammonium (TEA) 45. Therefore, CoCl treatment can alter rhodamine 123 efflux in HepG2 cells through multiple mechanisms involving mitochondrial function and transporter activity.
The mechanism by which CoCl affects rhodamine 123 efflux involves the inhibition of the drug efflux pump genes MDR1, FLU1, CDR1, and CDR2 in Candida albicans, leading to reduced drug efflux 46. The finding that CoCl treatment doubled the subpopulation of HepG2 cells capable of pumping out Rhodamine123 is consistent with previous research. Hirsch-Ernst reported that inhibitors of mdr1-dependent transport activity, which is involved in Rh123 efflux, can delay the accumulation of Rh123 in cells 29. Similarly, Bains (2005) demonstrated that increased P-glycoprotein (P-gp) transport of xenobiotics, such as Rh123, can increase cellular respiration rates 30. This finding suggests that CoCl treatment may increase the efflux capacity of cells, potentially affecting their ability to remove rhodamine123. However, further research is needed to fully understand the mechanisms underlying this effect.
CONCLUSION
CoCl effectively mimics hypoxia in HepG2 cells by inducing hypoxia-responsive gene expression and enhancing drug resistance mechanisms. However, the impact of CoCl is highly concentration dependent, with significant cytotoxic effects observed only at relatively high concentrations. These findings demonstrate that CoCl can be a valuable tool for studying hypoxia-related cellular responses and drug resistance in liver cancer cells. Nevertheless, careful consideration of the concentration and exposure time is essential to accurately interpret the effects and ensure the relevance of the experimental outcomes. Further studies are needed to elucidate the precise mechanisms and thresholds for CoCl2-induced hypoxic responses in HepG2 cells, optimizing its application in hypoxia research.
CONTRIBUTIONS
Chau-Huynh Dao Minh conducted the experiments to evaluate the results of real-time PCR analysis and investigate CoCl concentrations. Thu-Dang Pham Anh assessed drug resistance and performed the side population experiments. Dr. Sinh Nguyen conceived and designed the research, managed the research team, implemented the research plan, supported all the experiments, and wrote the manuscript.
ACKNOWLEDGEMENTS
The authors would like to express their gratitude to the Stem Cell Institute for financial support. We also extend our appreciation to the administrative team, including Ms. Khanh, Mr. Nghia, and Mr. Nhat at the Stem Cell Institute, for their invaluable assistance with nonscientific management tasks, which greatly facilitated the progress of this study.
Competing interests
The authors declare that they have no competing interests.

