Evaluation the effect of several anticancer drugs on Vietnamese breast cancer cells
- Stem Cell Institute, VNUHCM University of Science, Ho CHi Minh City, Viet Nam
Abstract
In Viet Nam, data from Conference of Cancer organized by the Ministry of Health has shown that breast cancer is the most popular cancer in women. Current mainly treatments are surgery, chemotherapy, and radiotherapy. However, the rate of recurrence after five years was very high. One of the causes of high relapse is cancer cells develop multidrug-resistant (MDR) thus reduced the efficiency of treatments. In this research, MTT assay was used for measured cell viability of Vietnamese breast cancer cells (VNBRCA cells) and positive control MCF-7 cell lines after treatment with several anticancer drugs as Doxorubicin (DOX), Tamoxifen (TAM), Mitomycin C (MMC) in 48h. After that, cancer cells were treated at haft maximal inhibitory concentration (IC50) of anticancer drug and observed cell morphology, apoptosis of cellular nuclear by AO/PI staining. IC50 value of VNBRCA cells with DOX, TAM, MMC were 0.641± 0.07 µM, 4.639 ± 0.933 µM and 1.338 ± 0.176 µM, respectively, which higher than IC50 of MCF-7 with DOX, TAM, MMC was 0.168 ± 0.037 µM, 7.085 ± 0.87 µM and 0.379 ± 0.159 µM, respectively. The response of VNBRCA cells with several anticancer drugs as DOX, TAM, and MMC was lower than the response of MCF-7, therefore, it showed that the specific features of VNBRCA cells; from which develop specific treatments for Vietnamese breast cancer patients.
Introduction
Breast cancer is the most frequently diagnosed cancer in Vietnamese women. In the last two decade from 2000 to 2010, the breast cancer incidence has more than double in age-standardized rate (ASR, the ratio per 100,000 people), particularly 13.8 in 2000 and 29.9 in 2010, approximately 12,533 new cases of breast cancer per year 1. In 2008, the ASR of 100,000 women in Hanoi and Ho Chi Minh City respectively 29,7% and 19.4% shown breast cancer still the most common cancer in Vietnamese women 2. These are many causes of breast cancer in Vietnamese women, such as the age of first menarche and menopause, number of children, using hormone and daily exercise 3. Breast tumors could be classified into many different subgroups according to their cell surface markers as the human epidermal growth factor receptor 2 (HER2), progesterone receptor (PR) or estrogen receptor (ER) 4. A survey on 492 breast cancer patients at the National Cancer Hospital of Vietnam in Hanoi from January 2007 to August 2013 showed that 61.6% of cases were ER-positive, 58.5% of cases were PR-positive, 21.4% of cases were Her2-positive, and 15% of cases were triple negative breast cancer (ER-negative, PR-negative, Her2-negative) 5. The other survival data from Hue Central Hospital and the Cancer Registry in Ho Chi Minh City showed the survival rates at 1 year, 3 years and 5 years following diagnosis were 0.94, 0.83 and 0.74 respectively; and the prognostic factor for mortality of breast cancer patients were stage at diagnosis, health status, marital status, using hormone, education level 6. Because severe problems of breast cancer, several methods were developed for the treatment, such as surgery, radiotherapy, chemotherapy, and hormone therapy. In cancer, surgery is the primary and most fundamental method both in the diagnosis and in cancer treatments. The type of surgery is based on cancer diagnosis, defined cancer states, remove a part or all of the tumor, e.g., the surgery in breast cancer treatment depends on the tumor location, size and other factors, the surgeon could use breast conserving or total mastectomy 7. Chemotherapy is an effective therapy against various type of human cancer, besides that many cancer cells also develop multidrug-resistant (MDR), the most problems for successful cancer treatment 8. Many drugs and therapies had been developed to cure breast cancer, such as Doxorubicin, Tamoxifen, Mitomycin C. Doxorubicin (DOX), is one of the most effective and broad-spectrum antibiotic, used in the treatment of a variety of hematology and solid malignancies, included leukemias, Hodgkin’s and non-Hodgkin’s lymphomas, breast, lung, ovarian and bladder cancers 9. 91011
Tamoxifen (TAM) is an antiestrogenic drug, which widely used for treating the patient who exhibits estrogen receptor-positive breast cancer 12. TAM is a prodrug form that could be metabolized in cytochrome P450 through CYP2D6 and CYP3A4 isoforms to the active form, which called endoxifen 131471516
Mitomycin C (MMC) is the broad-spectrum agent in tumor resistance that targets explicitly in CpG sequences. MMC was able to inhibit DNA synthesis through binding to the amino quinone group. MMC interacted with DNA could form the cross link between two complementary of the DNA strand, which could cause inhibited DNA synthesis and DNA replication 17.
However, breast cancer has become resistant to treatment, this means that chemotherapy or radiotherapy could not be affected on all cancer cells, most of the cancer cells may have killed, but some of them were either not changed and survive after treatments. Moreover, chemotherapeutic drugs are also toxic with normal cells, which caused several side effects to the patients. Therefore, the rate of tumor recurrence after five years in breast cancer patients still increase, maybe cancer cells had self-changed to resistant with a chemotherapeutic.
The purpose of this research is to investigate the effects of several common anticancer drugs in Vietnam breast cancer cells (VNBRCA) through evaluating the half-maximal inhibition concentrations (IC), cell viability of cells after anti-cancer drugs treatment.
Methods
Cell lines and chemicals
Human breast cancer cell line MCF-7 were purchased from the American Type Culture Collection. Vietnam breast cancer cells were supported by Stem Cell Institute, VNUHCM-University of Science, Viet Nam. Cancer cells were cultured in DMEM/F12 (Dulbecco’s Modified Eagle’s Medium/Ham F12) containing 10% FBS (Fetal Bovine Serum, Invitrogen, CA, USA), 1% antibiotic 100X (Invitrogen, CA, USA) at 37C with an atmosphere of 5%CO. Doxorubicin (DOX) was purchased from Sigma-Aldrich (44583, CAS number 25316-40-9) and Mitomycin C was purchased from Kyowa Hakko Kogyo Co were dissolved in aqueous, Tamoxifen was purchased from Sigma-Aldrich (T5648, CAS number 10540-29-1) and dissolved in dimethyl sulfoxide (DMSO). These anticancer drugs were used for MTT viability assay.
MTT viability assay
Cytotoxic effect on cancer cells was determined by using MTT assay after 48h treatment with anticancer drugs. Cancer cells were seeded onto 96-well plates at density 5.10 cells/well, cultured at 37C, 5% CO for 24h. Then, cancer cells were added with anticancer drugs at several concentrations 0 – 20 M and triple replicate in each concentration. Following incubation 48h at 37C 5% CO, added 10 of MTT solution (5 mg/ml) into 100 cell culture medium and incubated at 37C, 5% CO. After 4 hours incubation, treated plates were centrifuged 500g in 5 minutes, then removed the supernatant, added 100 of DMSO to each well and shaken for ten mins (horizontal shaker) to dissolve the violet formazan crystals. The optical density (OD) was measured by using Beckman Coulter DTX 880 Multimode Detector at 495 nm. The cytotoxic efficiency of anticancer drugs was specified by using the percentage of cell viability at several different concentration. The IC value was determined in the cell viability by GraphPad Prism 7.0 software. Each experiment was repeated in triplicate.
Cell viability (%) = Mean OD/Control OD x 100%
AO/PI staining assay
Acridine Orange/Propidium Iodine (AO/PI) are dyes which bind to nucleic acid and used to observe live/dead cells. Acridine Orange (AO) is a membrane permeable nuclear dye that stains all nucleated cells both in live and dead cells and emits the green fluorescence. While Propidium Iodine (PI) is membrane impermeable nuclear dye that stains dead cells to generate the red fluorescence. For AO/PI double staining, samples were combined with AO/PI Staining Working Solution at ratio 1:1, then cells were observed under a fluorescence microscope. Live cells will emit the green fluorescence (at the excitation 485 nm and the emission 548 nm), and dead cells will generate the red fluorescence (at the excitation 520 nm and the emission 590 nm).
Statistical analysis
The result of experiments was handled by using the GraphPad Prism 7.04 software. The data are performed in three independent experiments.
Results
The IC values (50% inhibitory concentration) of anticancer drugs in MCF-7 cells and VNBRCA cells
The cytotoxic efficiency of anticancer drugs as Doxorubicin, Tamoxifen, Mitomycin C against breast cancer cell lines was measured by using MTT assay. After 48h treatment with anticancer-drugs at different doses, cell viability of cancer cells was determined. Results of cytotoxicity assay were presented in Figure 1and

Cell viability of MCF-7 and VNBRCA1 after treatment with various concentration of anticancer drugs DOX (A, B), TAM (C, D), MMC (E, F).
DOX could inhibit the growth of MCF-7 cells and VNBRCA cells. These cancer cells were treated at the concentration of half dilutions 2, 1, 0.5, 0.25, 0.125 and 0.0625 µM for 48h and achieved inhibition of the cell growth compared with non-treated cells as a negative control. The cell viability following DOX treatment are shown in Figure 1A, the IC value of DOX against MCF7 cells and VNBRCA cells was measured from the cell viability by GraphPad Prism 7.0 (Figure 1B) as 0.168 ± 0.037 µM and 0.641± 0.07 µM, respectively (
To examine the inhibition of the growth on cancer cells, these MCF-7 and VNBRCA cells were treated with TAM at the concentration of half dilutions 20, 10, 5, 2.5, 1.25 and 0.625 µM for 48h, with non-treated cells as a negative control. The cell viability of TAM was shown in (Figure 1C), and the IC value of TAM was 7.085 ± 0.87 µM and 4.639 ± 0.933 µM in MCF-7 and VNBRCA cells, respectively (Figure 1D,
To investigate the inhibitory effect of MMC, these cancer cells were treated with anticancer drug MMC at the concentration of half dilutions 8, 4, 2, 1, 0.5 and 0.25 µM for 48h, with non-treated cells as a negative control. The result of MTT assay shows the cell viability following MMC treatment (Figure 1E), and the IC value in MCF-7 cells and VNBRCA cells (Figure 1F) respectively were 0.379 ± 0.159 µM and 1.338 ± 0.176 µM (
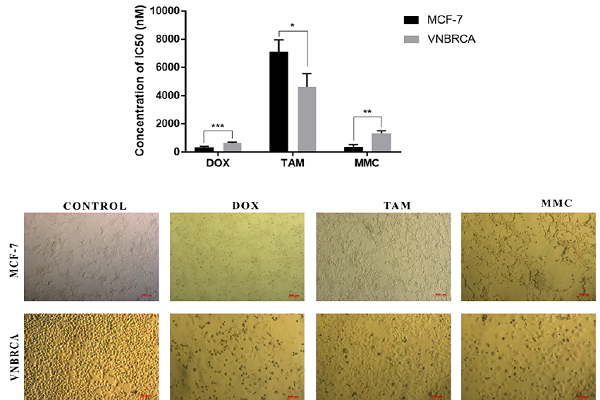
The IC50 value of both cell lines with anti-cancer drugs
|
MCF-7 |
VNBRCA1 | |||
|
IC50 (µM) |
Standard deviation |
IC50 (µM) |
Standard deviation | |
|
Doxorubicin |
0.168 |
0.037 |
0.641*** |
0.07 |
|
Tamoxifen |
7.085 |
0.87 |
4.639* |
0.933 |
|
Mitomycin C |
0.379 |
0.159 |
1.338** |
0.176 |
Breast cancer cells were treated with anticancer drugs at the IC values. The statistically different between two cell lines were shown in Figure 3. Morphological observation of cancer cells after treatment (Figure 2) showed the effect of cancer drugs on cell morphology in cell culture. In DOX and MMC treatment, the sizes of cells were larger than control, while in TAM treatment, cells were smaller, apoptotic cells had a round shape and floated in media culture.

Morphology of cancer cells was cultured with anticancer drugs at IC50 values.
Apoptosis of cancer cells after treatment with anticancer drugs at IC
Induction of apoptosis on cancer cells was also investigated by microscopic analysis of AO/PI double staining assay. Nuclei observation was performed to observe the efficiency of anticancer drugs which have the ability to induce apoptosis. Figure 3and Figure 4showed the untreated control and the anticancer drug-treated on MCF-7 and VNBRCA cells, respectively. With the green fluorescence, Acridine Orange (AO) showed all the nuclear cells, while in red fluorescence, only apoptotic cells could expose the light. Disintegrated nuclei appeared in the fluorescent images when cells were treated with anticancer drugs indicated that treated cells were undergoing apoptosis, while there were a few of dead cells in the control sample and those cells did not show the abnormal in nuclei DNA.

Fluorescent micrographs of AO/PI double-stained MCF-7 cells after 48h treatment with anticancer drugs.

Fluorescent micrographs of AO/PI double-stained VNBRCA cells after 48h treatment with anticancer drugs.
Discussion
Breast cancer is popular cancer in woman, despite the evolution of treatments, it is still the primary cause of death in female cancer patients. Doxorubicin, Tamoxifen, and Mitomycin C are a widely used drug for treating breast cancer patients. The primary mechanism in the response of cancer cells with chemotherapy is the activation of apoptotic pathways. The cell viability is an important parameter and is directly associated with the cytotoxic effects of a drug 19.
In this research, DOX and MMC caused apoptosis both in MCF-7 and VNBRCA cells, the IC values of DOX and MMC on VNBRCA cells were higher than on MCF-7 cells (
The cytotoxicity effect of TAM on VNBRCA cells were higher than on breast cancer cells MCF-7, so the IC value of TAM on VNBRCA cells was lower than on MCF-7 cells (
Conclusion
Vietnam breast cancer cells were less sensitive to anticancer drugs which evaluated in this work. MTT assay result and microscope images indicated that the cytotoxicity of the extract was dose-dependent. With DOX and MMC, VNBRCA cells were less sensitive than MCF-7 cells, means that high doses of these anticancer drugs would be treated in breast cancer patients, which would cause the high cytotoxicity and more dramatic side effects. With TAM, VNBRCA cells were more sensitive, produced by ER-positive cancer cells. Breast cancer patients need to identify cell surface markers, which could find the appropriate therapy, help to increase the effect of anticancer drugs, reduce the side effect of chemotherapy and the low cost of treatment. The primary cause of therapeutic failure in breast cancer patients is multi-drug resistance to cytotoxic chemotherapy, so using a reasonable dose of anticancer drugs may help to cure and prevent the tumor recurrence, improve the survival rate. Besides that, breast self-examination, screening programs, and new treatments should be encouraged and increased the life expectancy of Vietnamese breast cancer women.
Competing Interests
The authors hereby declare there are no conflicts of interest associated with this work.
Acknowledgments
Vietnam National University funded this experiment, Ho Chi Minh City, Viet Nam under grant number A2015-18-01/HD-KHCN and VNUHCM-University of Science, Ho Chi Minh City, Viet Nam with grant number T2017-42. The author also thanks to Stem Cell Institute for helping to complete this project.
Abbreviations
IC50: the half-maximal inhibition concentrations
MTT: 3-(4,5-dimethythiazol-2-yl)-2,5-diphenyltetrazolium bromide
DOX: doxorubicin
TAM: tamoxifen
MMC: mitomycin C

