Electrochemical performance of sulfone-based electrolytes in sodium ion battery with NaNi1/3Mn1/3Co1/3O2 layered cathode
- Key laboratory of Applied Physical Chemistry (APCLAB), VNUHCM-University of Science
- Department of Physical Chemistry, Faculty of Chemistry, VNUHCM- University of Science
- Department of Physical Chemistry, Faculty of Chemistry, VNUHCM- University of Science Key laboratory of Applied Physical Chemistry (APCLAB), VNUHCM-University of Science
Abstract
Introduction: Sulfolane (SL), having an edge of low melting point over other sulfones, has been adopted as an electrolyte co-solvent for lithium-ion battery (LIB), as it exhibits high stability against oxidation and combustion while not causing much side effects to the battery electrochemistry. It is therefore expected that SL may serve as a safety-enhancing agent in sodium-ion battery (SIB). To evaluate the effect of SL content on the behavior of common carbonate-based sodium electrolytes as well as the compatibility of SL-based electrolytes with NaNi1=3Mn1=3Co1=3O2 (NaNMC) cathode, mixtures of 0, 10, 20, 30 or 50% vol. SL and each of the following, EC:PC 1:1 vol. (EP11), EC:DMC 1:1 vol. (ED11), EC:PC:DMC 1:1:3 vol. (EPD113) and EC:PC:DMC 3:1:1 vol. (EPD311), with or without 1M NaClO4, were studied with regard to both inherent properties and performance in NaNMC half-cells.
Methods: Solvent flammability was evaluated via the self-extinguishing time (SET) and ignition time indexes. Conductivity and viscosity were respectively measured by Electrochemical Impedance Spectroscopy (EIS) and Ostwald method. Electrochemical techniques, i.e. Cyclic Voltammetry (CV) and Galvanostatic Cycling with Potential Limitation (GCPL), were used to test the sodium-ion battery performance.
Results: A moderate amount of SL (typically below 30% vol.) proved to enhance both electrolyte non-flammability and self-extinguishing behavior, while maintaining an acceptable compromising rate in viscosity and conductivity. Amongst 30%-SL electrolytes, EPD311-based ones allow the best Na+ diffusion when combined with NaNMC cathode in sodium half-cell configuration. The corresponding system gives satisfactory performance: initial specific capacity of 97 mAh.g-1, 92% capacity retention, and above 90% reversibility after 30 cycles at C/10 rate.
Conclusion: SL can be used as a stabilizing co-solvent for SIB, but its content should be limited to below 30% vol. to ensure its effectiveness.
Introduction
Sodium-ion battery (SIB) has recently emerged as a promising alternative to the prevailing lithium-ion battery (LIB), due to its better sustainability and suitability for large-scale applications, electric vehicles and grid storages1. Similar to its lithium predecessor, SIBs generally suffer from unguaranteed fire safety that arises from high volatility and flammability of the commonly used electrolyte solvents, organic carbonates2,3,4. Introducing a co-solvent with low vapor pressure and high burn-resistance, such as ionic liquids, sulfones and phosphates, proved to be a promising solution for this problem, as previously shown4,5,6.
Sulfone compounds are well-known for their excellent stability towards oxidation, including oxidative combustion. Besides, due to high polarity arising from the two S-O bonds, they are able to allow good salt solvation and high charge-transport number. And although sulfones are generally unable to form a protective layer on commonly-used graphitic anodes7, it was discovered recently that the use of appropriate anode binder, Li salt and electrolyte additive may help8,9. The only real limitation that prevents most sulfones from being attractive as an ambient-temperature electrolyte co-solvent for LIB (as well as SIB in the future) is their point.
Being one of the rare examples of low-melting sulfones, sulfolane (SL, also known as tetramethylene sulfone) has unsurprisingly received much interest from the LIB community, either as an electrolyte solvent, co-solvent or additive. As expected, SL exhibits desirable properties for a safe electrolyte solvent: wide liquid range (melting point T = 27.5C and boiling point T = 285C), high flash point (T = 165C) and high dielectric constant (ε = 60 at 25C). The stability-related advantages have also been well-demonstrated to be inheritable to SL-based electrolytes without much compromise in electrochemical capability. For example, 1M LiPF in SL:EMC 1:1 vol., while being about 20 times less flammable than its counterpart (EC:EMC 3:7 vol.), was still able to work well in a LiNiMnO/LiTiO full-cell, even after 1000 cycles at 2C rate 6. More recently, Kurc . 10 showed that solutions of various Li salts in SL solvent, with or without a small amount of vinyl carbonate additive, exhibited comparable flash point to SL and remained finely stable after 20 cycles working in LiNO half-cell at up to C/2 rate.
Considering the analogies between LIB and SIB, we expect that SL acts as a powerful co-solvent for SIB electrolyte. To evaluate the effects of SL on the behavior of carbonate-based sodium electrolytes and estimate the appropriate SL content, we investigated the mixtures of 0, 10, 20, 30 or 50% vol. SL with each of the four common carbonate combinations, namely EC:PC 1:1 vol. (EP11), EC:DMC 1:1 vol. (ED11), EC:PC:DMC 1:1:3 vol. (EPD113) and EC:PC:DMC 3:1:1 vol. (EPD311), either in the absence (applied in flammability tests) or presence (all other tests) of 1M NaClO. Important parameters of SL-contained electrolytes, including SET, ignition time, viscosity and conductivity, as well as their dependence of SL content were determined. We also managed to figure out a favorable range for SL content although the optimal value has yet to be concluded. The electrolytes with favorable SL content were then tested and compared in terms of electrochemical performance in NaNiMnCoO (NaNMC) half-cell.
Methods
Electrolyte and cathode composite preparation
Carbonate solvents including ethylene carbonate (EC), dimethyl carbonate (DMC), propylene carbonate (PC), sulfolane (SL) and NaClO were purchased from Sigma-Aldrich (St. Louis, MO, USA) with high purity (> 99.0%) and stored in glove box under argon atmosphere ([HO] < 10 ppm). Carbonate mixtures (EP11, ED11, EPD113 and EPD311) were first prepared by mixing the components, then mixed with 0, 10, 20, 30 or 50% vol. SL; 1M NaClO was finally added. At the end of each step, the mixtures were stirred for 8-12 hours.
NaNiMnCoO was synthesized by co-precipitation method. The hydroxide precursor NiMnCo(OH) was prepared by dripping 10 mL of 3M aqueous solution of Ni(NO), Co(NO) and Mn(CHCOO) following the stoichiometric ratio into 25 mL of 4M NaOH solution. The reacting system was kept at 50℃ and stirred at 500 rpm for 15 hours. The product was then filtered at low pressure and washed by distilled water until pH became neutral. The powdered NiMnCo(OH) was then dried under vacuum at 100℃ for 15 hours. A homogeneous mixture of hydroxide product and NaCO (5% excess) was calcined following a three-step solid state process: 500℃ for 6 hours, 900℃ for 36 hours, and then quenching immediately in Argon filled glove box.
Cathode composite was prepared by mixing 80% wt. NaNMC powder, 15% wt. carbon C65 (Timcal) and 5% wt. PTFE binder (Sigma-Aldrich). The resulting paste was laminated and then cut into 10-mm-diameter round disks. Both processes were carried out in glove box.

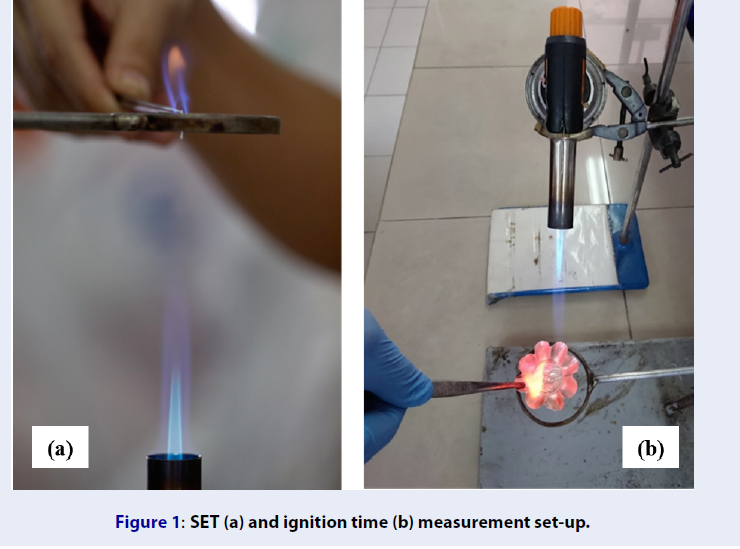
SET (a) and ignition time (b) measurement set-up.
Flammability test
All flammability tests were performed on electrolyte solvents (carbonate-SL mixtures, without salt) only. Solvent flammability was assessed via two parameters: the self-extinguishing time (SET) and the ignition time. In the SET measurement (Figure 1a), a fixed amount of solvent immobilized on a 14-mm-diameter piece of Whatman paper was exposed to a burner for 3 s at the distance of 13 cm to trigger ignition. The time the sample continues to burn after removal from the flame, the SET, was recorded and normalized against solvent mass (as proposed by Xu11). Regarding the ignition time measurement (Figure 1b), the solvent (100 μL, unless otherwise stated) was placed on a metallic container and ignited from the distance of 10 cm and the inclination angle of 45. vertical. The time it takes to form a sustainable flame was recorded and regarded as the solvent ignition time. All reported SETs and ignition times are average values calculated from the results of 5 experiments.
Conductivity and viscosity measurements
Electrolyte ionic conductivity was determined by Electrochemical Impedance Spectroscopy (EIS) recorded on Bio-Logic VSP3 instrument in the frequency range of 10 Hz to 1 MHz. Sample (0.5 mL each) were placed in a dip-type glass cell of known cell constant (CDC749 conductivity cell, radiometer, and distance between Pt electrodes (fixed at 4 mm). The samples were kept at the desired temperature for 120 minutes prior to measurement. Viscosity determination was conducted on an Ostwald CANON 150 viscometer (Canon, Tokyo, Japan). Sample temperature was adjusted by a controlled-temperature chamber.
Electrochemical analysis
Electrochemical techniques were performed on Bio-Logic MGP2 instrument using Swagelok half-cell with Na metal foil (Aldrich, battery grade) as anode, glass microfiber paper (Whatman, GF/D) soaked in one of the concerned SL-based electrolytes as separator, and as-prepared NaNMC composite as cathode. Cell assemblage was conducted in glove box.
Cycling Voltammetry (CV) was carried out in the voltage range of 2 V – 4 V . Na, at various scan rates ranging from 0.01 to 0.20 mV.s. From the slope of I (peak current) . v (square root of scan rate) plot, Na diffusion coefficient (D) values were calculated using Randles-Sevcik equation:
where I is the peak current (A), n is the number of charge transferred, A is the electrode area (0.785 cm), D is Na diffusion coefficient (cm.s), C is the Na concentration of the cathode (mol.cm), and v is the scan rate (V.s). Cycling test was performed at C/10 rate and also in the voltage range of 2 V – 4 V . Na.
Results
Figure 2 expresses the dependence of solvent SET values upon SL content. In general, with the addition of SL, SET values initially decreased to reach a minimum at around 20% to 30% vol. SL, before sharply rising up. This suggests that while SL, at a reasonable content, does exhibit flame-retardant effects, its presence in excessive amount may be detrimental to the solvent self-extinguished behavior. It was also noted that SET values of DMC-rich solvent families, ED11- and EPD113-based ones, tended to be lower than those of other families.

SET of carbonate-SL solvent families at various SL contents. The self-extinguishing nature of electrolytes is enhanced when a small amount of SL is added. However, when exceeding 20-30% vol., SL may promote the electrolyte flame sustainability due to its heat-economical combustion.
Ignition time of pure SL seems not to depend on the sample amount and is much larger than carbonate solvents
| SL vol. (μL) | 100 | 200 | 300 | 400 | 500 |
|---|---|---|---|---|---|
| Ignition time (s) | 10.23 ± 0.26 | 9.77 ± 0.35 | 10.25 ± 0.35 | 10.52 ± 0.22 | 10.24 ± 0.46 |
| Mean ignition time: 10.22 ± 0.38 s | |||||
The ignition time values of pure SL are shown in

Ignition time of carbonate-SL solvent families at various SL contents. Electrolytes that are rich in SL or cyclic carbonates (EC and PC) are generally more difficult to ignite. The increase in ignition time with SL content, however, is not simply linear.

Viscosity η (a) and ionic conductivity σ (b) of carbonate-SL electrolyte families at 350C
Figure 4 shows the viscosity and ionic conductivity at 35C of various carbonate-SL electrolytes as a function of their SL content. In all cases, the viscosity exhibits a positive correlation towards SL content, while the ionic conductivity, as expected, follows an opposite trend. Another point worth considering is that despite not standing out in terms of fluidity, 1M NaClO in EPD311 + SL demonstrates good ionic conductivity, perhaps amongst the best ionic conductivity of interested electrolyte families.
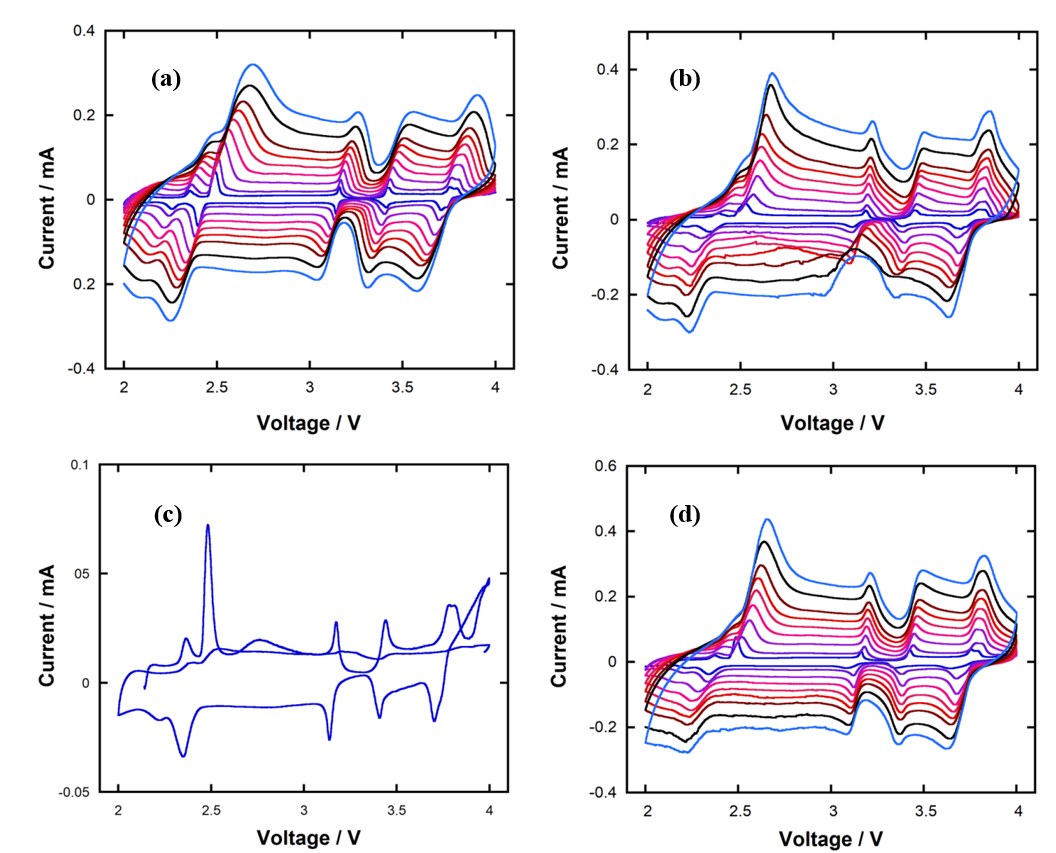
CV curves of NaNMC half-cell using 1M NaClO4 in mixture of 30% vol. SLand (a) ED11, (b) EP11, (c) EPD113 and (d) EPD311, as electrolyte. Scan rateswere 0.01, 0.02, 0.04, 0.06, 0.08, 0.10, 0.12, 0.16 and 0.20 mV.s-1.The peak names (Ip,a1, Ip,c1, …) are shown for thepurpose of peak identification. Except EPD113-based electrolyte, the otherswork well with NaNMC material.
Diffusion coefficient of Na+ ion in NaNMC half-cells employing 30%-SL electrolytes.1M NaClO4 was used as electrolyte solute in all cases. EPD311-basedelectrolyte generally allows the most effective Li diffusion
| Electrolyte solvent | 1013 DNa (cm2.s-1) | |||||||||
| Ip,a1 | Ip,c1 | Ip,a2 | Ip,c2 | Ip,a3 | Ip,c3 | Ip,a4 | Ip,c4 | Ip,a5 | Ip,c5 | |
| ED11 + 30% SL | 0.68 | 1.8 | 11 | 12 | 0.73 | 2.5 | 5.5 | 1.2 | 4.6 | 12 |
| EP11 + 30% SL | 1.0 | 0.33 | 14 | 11 | 0.94 | 7.4 | 6.7 | 1.9 | 5.3 | 16 |
| EPD311 + 30% SL | 0.71 | - | 20 | 12 | 1.6 | 9.0 | 9.6 | 2.3 | 7.1 | 18 |
The ability of 30%-SL electrolytes to facilitate Na intercalation kinetics in NaNMC half-cell was tested to provide a preliminary evaluation of their feasibility in SIB. Figure 5 shows the multi-scan-rate CV curves of NaNMC cathode in our 30%-SL electrolytes. Except for the EPD113-based system, which decomposed only after the first scanning cycle, the other three electrolytes are compatible with NaNMC material as their CV profiles reveal clear and relatively reversible redox peaks. That being said, because EPD311-based electrolyte allows highest Na diffusion coefficient at most redox events, as evidenced in
Voltage
DISCUSSION
The addition of SL has significant impacts on the overall behavior of traditional carbonate-based electrolytes. On the one hand, SL can greatly reduce the solvent flammability and, thus, the battery fiery hazards, if its content lies within a specific range (around 30% vol.). Considering SL low volatility and flammability, it is expectable that increasing SL content results in better SET and ignition time indexes. Although this is mostly the case at low SL content, one should notice that the solvent self-extinguishing nature started to decline when the SL content exceeds a threshold value and is presumably large enough for the combustion of SL to be triggered. It is likely that flame-resistant substances, such as SL, EC and PC, are able to sustain their flame for a long time once they get ignited, as their combustion rate is reasonably low and the heat loss during combustion is thus limited. In this way, the aforementioned low SET values of DMC-rich solvents as well as other similar observations reported in previous studies3,4 can also be explained. On the other hand, SL inevitably thickens the electrolyte solutions and, as a result, compromises their ionic conductivity to a certain extent. However, the conductivity loss corresponding to the addition of up to 30% vol. SL remains at around 20%-30%. We believe that such a sacrifice is practically acceptable and may barely interfere with the battery performance, given that the rate determining step of Li intercalation process is usually the diffusion through the cathode-electrolyte interface (CEI) and/or within the solid electrode, rather than the ionic conduction in liquid phase. In brief, the results of flammability tests as well as viscosity and ionic conductivity measurements suggest that the addition of a moderate SL amount, below 30% vol., is generally favorable to improve the safety profile of our electrolytes.
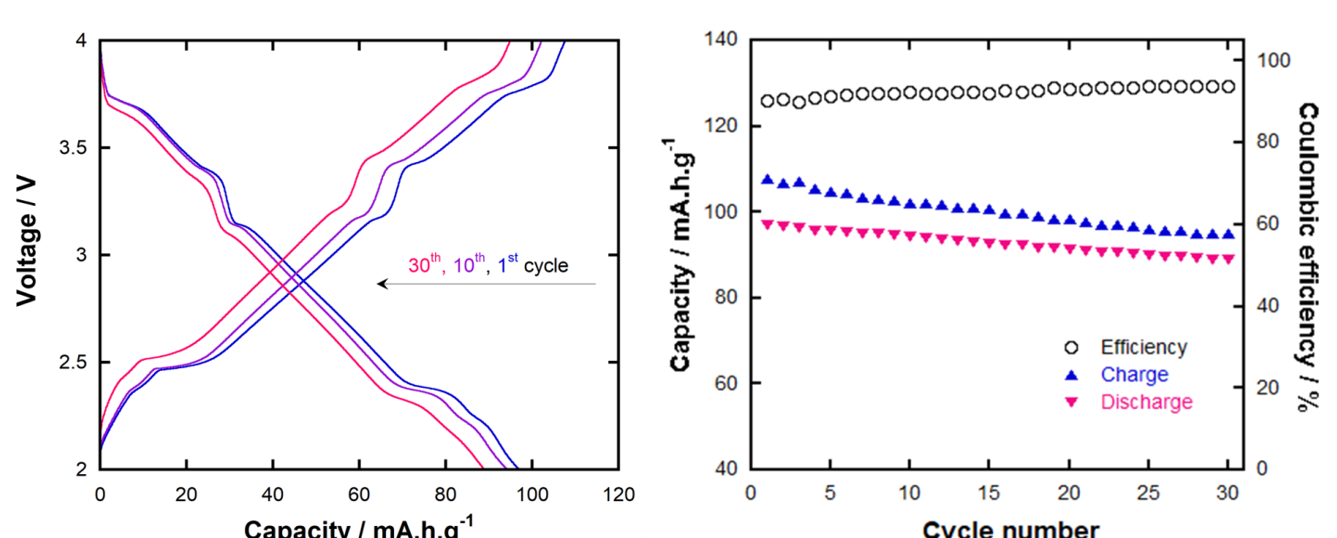
Amongst tested electrolytes, the EPD113-based one is the one with the most subjects, as well as the only one that underwent oxidative decomposition during cycling test with NaNMC material. Although high DMC content clearly signifies the low anodic stability of EPD113-based electrolyte, its oxidation at such a low voltage as 4 V . Na/Na is unexpected and may result from direct exposure to the catalytic transition metals in cathode material. A comparison between Na diffusion coefficients in the other three systems reveals that the EPD311-based is the most compatible with NaNMC material, suggesting that either too low or too high DMC content in the electrolyte (as in EP11- and ED11-based ones, respectively) is not ideal in terms of promoting Na diffusion kinetics. The underlying reason has yet to be fully investigated, but we believe that it can be associated with the effects of different CEI behaviors. Cycling test results confirm that the EPD311-based electrolyte/NaNMC half-cell works well at regular cycling rate to give typical NaNMC charge-discharge profile as well as high specific capacity, capacity retention and cycling reversibility.
CONCLUSIONS AND PERSPECTIVE
Carbonate-SL electrolytes were investigated in terms of their inherent properties as well as their electrochemical performance in NaNMC half-cell. In general, increasing SL content in the range of 0-30% vol. proportionally reduces the electrolyte fire hazard at an acceptable expense of conductivity drop, based on the SL-case. SL compromises both the battery safety and performance aspects. Amongst 30%-SL electrolytes, the EPD311-based one exhibits the best compatibility with NaNMC material. Their combination operated smoothly at C/10 rate, yielding 97 mAh.g discharging capacity, above 90% reversibility and 92% capacity retention after 30 cycles. It is suggested to test the compatibility, including interfacial electrochemistry, Na intercalation kinetics and cycling performance, of carbonate-SL electrolytes towards SIB anode as well as other cathode materials. This helps to ensure and diversify their applicability in full SIB cells.
ABBREVIATIONS
SL: sulfolane
SIB: sodium-ion battery
LIB: lithium-ion battery
EC: ethylene carbonate
PC: propylene carbonate
DMC: dimethyl carbonate
EP11: EC:PC 1:1 vol.
ED11: EC:DMC 1:1 vol.
EPD113: EC:PC:DMC 1:1:3 vol.
EPD311: EC:PC:DMC 3:1:1 vol.
NaNMC: NaNiMnCoO
SET: self-extinguishing time
D: diffusion coefficient of Na ion
CEI: cathode-electrolyte interface
COMPETING INTERESTS
The authors declare that there is no conflict of interest regarding the publication of this article.
AUTHORS’ CONTRIBUTION’S
All the authors contribute equally to the paper including the research idea, experimental section and written manuscript.
ACKNOWLEDGEMENT
The authors acknowledge funding from Viet Nam National University of Ho Chi Minh City (VNU-HCM) under the project number C2019-18-08.

