Effects of L-ascorbic acid or insulin-transferrin-selenium on in vitro maturation and energy metabolism on the developmental competence of porcine parthenogenetic embryos
- Cellular Reprogramming Laboratory, School of Biotechnology, International University, Ho Chi Minh City, Vietnam
- Vietnam National University, Ho Chi Minh City, Vietnam
Abstract
Introduction: Porcine parthenogenetic embryos have emerged as promising tools in biomedical science. However, in vitro maturation and in vitro development often lead to elevated levels of reactive oxygen species and reduced developmental competence of the embryo. This study focused on advancing our understanding of how to increase the developmental competence of embryos. In addition, the embryos of large animals have specific metabolic pathways, and their energy substrate requirements for preimplantation are also more flexible than those of rodents. Little is known about the metabolism of porcine embryos from the 4-cell stage until morula. This study examines the effects of adding insulin-transferrin-selenium and/or L-ascorbic acid to the medium for maturing prepubertal porcine oocytes and establishes a new strategy of using glucose and pyruvate/lactate in porcine embryos cultured for standard protocols.
Methods: The effects of insulin-transferrin-selenium and/or L-ascorbic acid during in vitro maturation were examined in experiment 1. In experiment 2, porcine parthenogenetic embryos were cultured in energy-free medium (days 0--5, 1--5, and 2--5, respectively) for the remaining duration in medium containing lactate-pyruvate or glucose for days 0--5 to investigate the effects of energy substrates in early developmental stages. Finally, the strategies of using glucose and lactate-pyruvate for in vitro development in Experiment 3 were determined, and the blastocyst quality and quantity were evaluated by culturing in different combinations of energy substrate and culture durations: (1) glucose (7 days); (2) lactate-pyruvate/energy-free/glucose (2 days/3 days/2 days, respectively); (3) lactate-pyruvate (7 days); and (4) lactate-pyruvate/glucose (2 days/5 days, respectively).
Results: Supplementation with a combination of insulin-transferrin-selenium and L-ascorbic acid during in vitro maturation significantly improved the quantity and quality of blastocysts, whereas insulin-transferrin-selenium alone did not have a significant effect. Moreover, the embryos were capable of self-growth without an energy substrate throughout the preimplantation stages, resulting in late cleavage and 2-cell block. The exposure of the embryo to energy substrates for at least the first 48 hrs tended to have greater beneficial effects on embryo developmental competence. In addition, compared with glucose, exposure to lactate and pyruvate significantly increased both embryo quantity and quality.
Conclusion: Adding a combination of insulin-transferrin-selenium and L-ascorbic acid to in vitro maturation medium could increase the developmental competence of porcine parthenogenetic diploid embryos. Survival and development at the 1-cell stage are energy dependent, but those from the 4-cell stage to the early blastocyst stage are energy independent. The morula-early blastocyst formation with lactate-pyruvate had a better result than those exposed to glucose only.
INTRODUCTION
Parthenogenesis is a process in which zygotes are produced without the presence of sperm. Owing to the lack of a paternal genome, parthenogenetic embryos are unable to achieve full-term development. This unique phenomenon has the potential to address ethical concerns related to human embryos. In addition, the anatomical and physiological characteristics of pigs are comparable to those of humans, making them more suitable for clinical-translational research. They have been utilized in alternative human models supporting biomedical research, bioorgans, and new drug testing trials. Recently, porcine embryo efficiency has improved because of the benefit of model research. However, producing large numbers of blastocysts remains a challenge. Biochemical and morphological changes during embryonic culture are related to changes in the nutritional and energy requirements of developing embryos, including zygotic gene activation, morula compaction, and blastocyst formation and expansion. Specifically, the developmental competence of mature oocytes is usually impaired due to oxidative stress 1. High reactive oxygen species (ROS) production can cause oxidative stress, which induces apoptosis. Therefore, the reduction in oxidative stress caused by ROS production could improve the cytoplasmic maturation of porcine oocytes 2. L-ascorbic acid (LAA) is an essential micronutrient known for its ability to moderate a variety of biochemical processes and has both antioxidant functions and enzymatic cofactor functions; it is a redox catalyst that can reduce and neutralize ROS3. In addition to suppressing ROS reactions, oocytes require nutrients to improve their quality before reaching the MII stage. Insulin-transferrin-selenium (ITS) is preferred as a supplement in most serum-free cultures because it enhances cell proliferation and survival and decreases the serum requirements of many cell types. It also increases both quantity and quality in several maturation (IVM) systems and during porcine embryonic development 4. Insulin helps increase the absorption rate of nutrients instead of increasing the concentration of nutrients supplied to the medium5, whereas transferrin and selenium have antioxidant properties. However, the effects of either LAA or ITS on porcine oocytes are not completely understood.
Moreover, the embryo culture medium partially affects the survival rate and quality of the embryos . Energy metabolism is crucial for embryo development because it involves high energy demands from different substrates. In the context of preimplantation embryos, it is important to focus on the major substrates added to embryo development (IVD) culture media. It has been reported that rats, rabbits, and monkeys prefer lactate and pyruvate as the central energy sources required for embryos6, whereas glucose inhibits embryonic development in hamsters and cattle but is not obligatory for pigs7. Among porcine embryos, North Carolina State University-23 (NCSU-23) and Porcine Zygote Medium 3 (PZM3) variants are currently the most commonly used media for porcine embryo culture. PZM3 contains 2.73 mM lactate and 0.17 mM pyruvate, and NCSU23 contains 5.56 mM glucose but lacks pyruvate and lactate. Previous studies have shown that pyruvate and lactate are essential sources of carbohydrates during the early stages of preimplantation development 8, and glucose becomes a key energy source after compaction9. Although many studies have suggested that the energy substrate should be altered during embryonic development 10, current reports have only used one energy substrate addition because no differences have occurred during development 11. Moreover, oocytes can provide enough substrates for energy production necessary for the initial days of embryo development12, and -produced porcine zygotes have been demonstrated to complete preimplantation embryonic development without energy requirements. While characteristic metabolites have been defined for the 2-cell and morula stages, the metabolic characteristics of the 4-cell stage, a critical stage for detecting synchronous embryos that lead to abortion, have not been fully documented.
Here, we first examined the effect of ITS, specifically LAA, on the maturation of porcine oocytes. The second objective of this study was to clarify whether porcine embryos require energy substrates, especially at the 4-cell to morula stage. The third objective was to provide an overview of all the strategies of using specific-energy substrate supplementation at different times of culture on the developmental competence of parthenogenetic diploid porcine embryos. This thorough documentation of energy metabolism and embryo behaviors during IVM and IVD sets the stage for further improvements in embryo culture conditions and the optimization of viability assays. This may provide additional information for discriminating between the suitability of different substrates for embryo development, which can improve the knowledge of the production of embryos.
MATERIALS AND METHODS
Experimental Design
Experiment 1: Effects of ITSs during maturation and culture on embryo production and quality
In this study, we evaluated whether the combination of ITS and LAA affects embryo production during IVM and culture. These groups included (1) the control oocytes, n=59; (2) the oocytes supplemented with ITS in the IVM medium for 24 hrs (+ITS), n=81; (3) the oocytes supplemented with L-AA in the IVM medium for 24 hrs (+L-AA), n=102; and (4) the oocytes supplemented with a combination of ITS and L-AA in the IVM medium for 24 hrs (+ITS-LAA), n=134. The effects of supplementation on the culture were examined via the cumulus expansion and development rates at the 2-cell, 4-cell, 8-cell, morula, and blastocyst stages, as well as the total cell number in blastocysts as a measure of embryo quality.
Experiment 2: Evaluation of the energy substrate-dependent or energy substance-independent duration of embryo development during the first 5 days after parthenogenesis activation
Oocytes were cultured in EFree medium for 0–5 days, 1–5 days, or 2–5 days in the presence of Glu or LP for 24 hrs or 48 hrs in the gaps, respectively. One-cell parthenogenetic embryos cultured with only LP or Glu for the whole culture period were chosen as the control group. The cleavage rates on day 2 and morula-early blastocysts, blastocyst rates, sizes and total cell numbers on day 5 were evaluated.
Experiment 3: Effects of different energy supplementation strategies on energy substrate independence during embryo development
The effects of all the strategies and the modified combination media at different culture durations on the development of porcine parthenogenetic embryos were examined. After activation (day 0), a total of 4 different IVD groups were used: (1) Glu (0-7 days), n=433 (2) LP D0-2/E-Free D2-5/Glu D5-7 (2 days/3 days/2 days, respectively), n=327; (3) LP (0-7 days), n=244; and (4) LP D0-2/Glu D2-7 (2 days/5 days, respectively), n=151. All the embryos were cultured until day 7, and the development rate to the blastocyst stage and the number of cells per blastocyst were assessed.
The experimental design schematic is shown in Figure 1.

Schematic representation of the experimental design. Experiment 1: The effects of the combination of ITS and LAA supplementation on embryos were were evaluated during in vitro maturation. Experiment 2: Effects of the substance-independent duration of embryo development during the first 5 days after parthenogenesis activation. Experiment 3: Evaluation of different energy supplementation strategies during in vitro embryo development.
Oocyte collection and maturation (IVM).
Porcine ovaries were collected from the slaughterhouse and transported to the laboratory while they were kept in phosphate-buffered saline (PBS). The ovaries were rinsed several times with PBS, and the oocytes were collected from 4–6 mm follicles via the dissection method. Only cumulus‒oocyte complexes (COCs) with multiple layers of intact cumulus cells, granulosa cells, and uniform ooplasm were selected for subsequent IVM. The detailed procedure of IVM was described in a previous study13. Briefly, the maturation medium consisted of tissue culture medium (TCM-199) supplemented with 10% (v/v) porcine follicular fluid (pFF), 10% (v/v) fetal bovine serum (FBS), 10 IU/mL human chorionic gonadotropin, and 0.91 mM sodium pyruvate, with supplementation of 3 treatment groups: 50 µg/mL LAA, 1% (v/v) ITS, or ITS-LAA for the first 22 hrs during 44 hrs of IVM. The COCs of the three treatment groups were placed in separate IVM floating drops (10–15 COCs/100 µL) covered with mineral oil. The microdroplets were stabilized at 38.5°C in an atmosphere of 5% CO before culture.
Parthenogenetic activation of diploid embryos
The procedure of parthenogenetic activation was described in a previous study 14. Briefly, after 44 hrs of maturation culture, denuded mature oocytes with the first polar body were selected. Recovered mature oocytes were activated by a single direct-current (DC) pulse of 100 V in 100 msec twice, with 5 s intervals between two pulses by an Electro Cell Fusion LF201 (Nepagene, Japan) in a medium containing 0.3 mM mannitol, 0.1 mM MgSO4, 0.05 mM CaCl, and 0.01% (w/v) PVA. After activation, the oocytes were cultured in NCSU23 supplemented with 5 µg/mL cytochalasin B (CB) for 6 h to produce porcine parthenogenetic diploid embryos. The embryos were then washed to remove CB and continuously cultured in the following treatments.
Combined effects of lactate/pyruvate (LN), energy-free (EFree), and glucose (Glu) on the development (IVD) of porcine parthenogenetic embryos
Embryos were allocated randomly to 3 groups of culture media: (1) basic NCSU23 media containing 0.55 mM glucose (Glu), (2) modified NCSU23 with 0.55 mM Glu replaced with 2.73 mM sodium lactate and 0.17 mM sodium pyruvate (LP), and (3) modified NCSU23 with free-energy substrates supplemented with NaCl for osmolarity rebalancing (EFree). To examine the combined effect of the energy supply medium, the embryos were cultured in 4 groups: (1) Glu (7 days), (2) LP/E-Free/Glu (2 days/3 days/2 days), (3) LP (7 days), and (4) LP/Glu (2 days/5 days). Each group was cultured in an atmosphere of 5% CO in humidified air at 38.5°C.
Quantitative and qualitative analysis
The embryo development rate was calculated by dividing the number of embryos reached in the expected stages by the number of MII oocytes or 2-cell embryos. The number of cells in the blastocysts on days 5 and 7 in each treatment group was detected via 4’,6-diamidino-2-phenylindole (DAPI) staining, as described in our previous report 15.
Statistical analysis
Each treatment was replicated at least three times, with each replication consisting of 15 or more oocytes or embryos. One-way analysis of variance (ANOVA) was performed via the Statistical Package for Social Statistics version 20 (SPSS). A value < 0.05 was considered statistically significant. The data are presented as the mean ± standard error of the mean (SEM).
RESULT
Effect of L-ascorbic acid and Insulin-transferrin-selenium during maturation of porcine oocytes
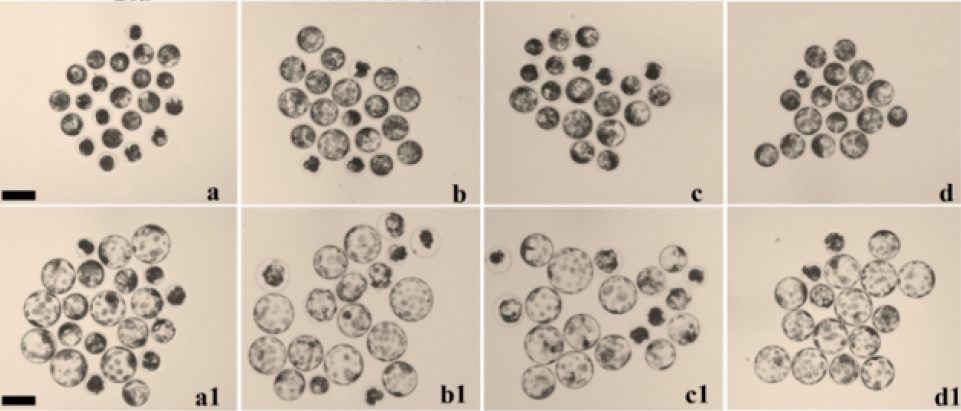
The viability of cumulus cells and oocytes is highly important in the assessment of oocyte maturation. In our experiment, COCs were subjected to either 50 µg/mL LAA or 1% (v/v) ITS during the first 22 hrs of IVM. The findings indicated that treatment of porcine COCs with both LAA and ITS resulted in greater expansion and fewer dead clumps of surrounding cumulus cells (Figure 2A). Although the addition of LAA alone or combined with ITS (LAA-ITS) did not affect the cleavage rate at the 2-cell stage compared with that in the control groups, it markedly improved embryonic development, particularly at the 4-cell stage (87.46±1.30%, 89.45±0.34% vs. 76.67±1.67%, p<0.05, respectively) and the blastocyst stage (45.87±1.30%, 49.32±1.38% vs. 33.89±0.56%, p<0,05, p<0.01, respectively). In contrast, ITS alone did not significantly improve development (Figure 2BC). In addition, supplementation with the combination of LAA and ITS resulted in a significant increase in the total cell number of day 7 blastocysts compared with that of the control groups (35.79±1.86 vs. 27.31±1.26, p<0.05, respectively), indicating significantly greater quality (Figure 2D).

Effects of LAA and ITS on the maturation of porcine oocytes. (A) Expansion of cumulus cells after 44 hrs of IVM in the control (nontreated, a), with and without either LAA or ITS (b, c) and combined LAA/ITS groups (d). (B) Day 7 expanded blastocysts from different groups are shown (a, b, c, d). (C) The development rates of 2-cell, 4-cell, 8-cell, morula, and blastocyst embryos from parthenogenetically activated mature oocytes were recorded. (D) Quality of embryos in terms of cell number. The data are presented as the mean ± SEM. Values with different letters within each developmental stage are significantly different (P < 0.05). A, Scale bar = 500 µm. B, Scale bar =100 µm.
Effects of energy substrates on the early development of porcine parthenogenetic embryos
In this series of experiments, embryos were cultured in basic medium (NCSU23) at different stages from pronuclei (day 0) to 4-cell (day 2) embryos with minor modifications in energy substrates, including the presence of glucose (Glu) or lactate/pyruvate (LP), and during the remaining gap periods, the embryos were cultured in energy substrate-free medium (E-Free), as shown in Figure 3 and

Dependences of
Dependence of
|
Medium |
Duration |
No. of 2-Cell |
Day 5 |
No. of cells |
Size of early blastocyst (µm) | ||
|
Basic |
EFree |
No. of MEB (%) |
Ratio of M/MEB (%)* | ||||
|
Glu |
DAY 0-5 |
- |
87 |
69 (79.35±1.40)a |
30/69 (43.56±2.29)a |
24.54±1.77a |
143.43±3.04ab |
|
DAY 0-1 |
DAY 1-5 |
77 |
64 (83.27±1.02)ab |
27/64 (41.58±3.17)a |
26.61±1.38a |
149.14±5.36b | |
|
DAY 0-2 |
DAY 2-5 |
77 |
63 (81.62±1.36)a |
24/63 (38.54±2.17)a |
25.46±1.59a |
147.45±4.36b | |
|
LP |
DAY 0-5 |
- |
98 |
90 (92.29±2.68)c |
7/90 (7.75±0.56)b |
30.74±1.72b |
170.13±6.12c |
|
DAY 0-1 |
DAY 1-5 |
71 |
64 (89.60±2.10)bc |
15/64 (23.07±2.25)c |
28.07±1.22ab |
157.60±4.12d | |
|
DAY 0-2 |
DAY 2-5 |
82 |
77 (93.57±1.86)c |
8/77 (10.58±0.75)b |
31.41±2.04b |
174.12±6.14c | |
|
EFree |
- |
DAY 0-5 |
76 |
34 (44.75±2.41)d |
27/76 (79.58±1.43)d |
17.05±1.13c |
134.29±1.83a |
In addition, the results revealed that the absence of energy substrates from the 2-cell (day 1) stage did not affect morula-early blastocyst (day 5) formation within the same energy groups (
This finding indicated that-produced embryos could develop independently from energy substrates. However, during normal development, porcine embryos require energy substrates at the 1-cell stage and could be independent from the 4-cell stage to the early blastocyst stage without detrimental effects. Moreover, LP was superior to glucose at the early stages of embryogenesis in terms of quantity, quality, and blastocoel expansion.
Effects of different media combinations on the development of porcine parthenogenetic embryos
To evaluate the effects of different energy substrate-dependent periods, porcine parthenogenetic embryos were cultured for 7 days under four conditions: 1) Glu-only, 2) LP-only, 3) LP/EFree/Glu (2 days, 3 days/2 days, respectively), and 4) LP/Glu (2 days/5 days, respectively). The results are shown in Figure 4.

Dependences of
In this study, the development rate from morula to early blastocyst formation (Figure 4AB) and the number of cells per blastocyst (Figure 4CD) were evaluated and compared among the groups. Compared with those in the Glu-only group, the development rates and cell numbers in the LP-only, LP/EFree/Glu, and LP/Glu groups were significantly greater. The LP-only, LP/EFree/Glu, and LP/Glu groups presented comparable results, with no significant differences. Compared with the Glu-only group, all the LP groups presented considerable effects on both the development rate and cell number, suggesting that these treatment groups presented increased blastocoel expansion capability and blastomere quantity.
This finding revealed that lactate and pyruvate are crucial for early embryonic stages because they induce development from the zygote to the 4-cell stage and that the absence of these substances may have detrimental effects on embryos at this stage.
DISCUSSION
We found that adding ITS-LAA during IVM resulted in the greatest improvement in the quantity and quality of the produced embryos. This led to a greater blastocyst rate and greater number of blastocyst cells than did the addition of L-AA alone, and the presence of ITS did not affect embryo development. In addition, this study revealed a high incidence of delayed cleavage and 2-cell block when embryos were cultured in a medium lacking energy substrate. Our results emphasize the critical role of energy substrates utilized by embryos from the zygote stage to the 2-cell stage in the subsequent development of the embryo.
Our previous study demonstrated that LAA could improve the developmental competence of porcine embryos from growing oocytes14. Moreover, supplementation with various nutrient sources, such as FBS 16, glucose17, cysteine18, and amino acids 19, can aid in improving the quality and development of oocytes during maturation. An excessive supply of nutrients may adversely affect oocyte viability and maturation. Therefore, ITS can mitigate these issues by enhancing hormone absorption. 20 and the synthesis of protein, thereby increasing oocyte metabolism21. However, an increase in metabolism is associated with the production of reactive oxygen species (ROS), and redox reactions affect key enzymes associated with metabolic pathways 22. ROS have been identified as major factors in the decline in oocyte quality and development23. The accumulation of ROS in the oocyte culture leads to lipid peroxidation of the cell membrane, DNA fragmentation, disruption of RNA transcription and protein synthesis 24, developmental blockage, and ultimately, cell death 1. L-ascorbic acid supplementation during IVM could decrease the level of reactive oxygen species (ROS) due to its antioxidative effect, resulting in improved cytoplasmic maturation by promoting the storage of glutathione (GSH) in oocytes and subsequent embryonic development 25. Therefore, the results suggest that the mutual support of LAA and ITS leads to an improvement in the blastocyst formation rate and the total cell number in the blastocyst.
The presence of glucose in IVF culture media at the zygotic stage in mice has been shown to alleviate oxidative stress thus, high-quality 8-cell embryos can develop26 and reach the morula stage27. In addition, pyruvate has been proven to have the ability to protect against cytotoxicity, and lactate acts as a potent cytosolic reductant in early embryos through lactate dehydrogenase28. It can also protect fragile cleavage-stage cells from oxidative stress and injury by regulating excessive glucose consumption.
When glucose or lactate and pyruvate were added to the IVD medium after electroactivation, the presence of lactate and pyruvate had greater beneficial effects than did the addition of glucose, at least for the first two days. It has been reported that precompacted embryos utilize pyruvate and lactate to fuel carboxylic acid-based metabolism via the Krebs cycle and oxidative phosphorylation to consume glucose via the Embden–Meyerhof pathway 29. Moreover, at the early stages of porcine embryonic development, small amounts of carbohydrates are necessary to provide oxaloacetate to prime the TCA cycle if the free fatty acids formed from triglycerides are oxidized. The enzyme pyruvate carboxylase can produce oxaloacetate from pyruvate. During the later developmental stages, porcine embryos obtain pyruvate from glucose via the glycolytic pathway 30. Thus, it is possible to supply pyruvate, which helps to produce oxaloacetate and is required to prime the TCA cycle.
Pyruvate has been shown to play a crucial role as an energy substance during early mammals embryonic development31, and LP has been identified as an important energy supplement in porcine embryos, particularly for early embryonic development . In our study, embryo culture with the combination of pyruvate-lactate and glucose during the early stages of development resulted in a significantly greater developmental rate in the morula and expanded blastocyst stages than did those cultured with glucose alone. This can be attributed to the fact that 91–97% of the ATP produced during embryonic development is derived from oxidative phosphorylation through the consumption of lactate/pyruvate, and the remaining ATP is produced in the glycolytic pathway associated with glucose consumption after the morula stage 32.
Previous studies have demonstrated that energy substrates are essential for massive cell proliferation of embryos from the morula to early blastocyst stages and that lactate/pyruvate (LP) may partially reflect the superiority of PZM3 over the NCSU23. However, our results revealed that when energy substrates were absent from the 4-cell stage to the early blastocyst stage, the quantity and quality of the embryos were similar to those when energy substrates were present. These findings indicate that porcine embryos may not depend on energy substrates during this period and that the energy substrates used by embryos from the zygote to the 2-cell stage are crucial for the later development of the embryo.
CONCLUSION
To summarize, the combined supplementation of LAA and ITS in the culture system of porcine oocytes enhanced maturation and developmental competence. The results provide insights into the independence of energy substrates in the development of embryos and the effects of specific energy substrate supplementation during different culture periods. This study broadens our understanding of embryonic metabolism in the context of a broad, interconnected network of metabolic mechanisms that influences viability. A thorough understanding of embryonic metabolism is crucial for enhancing embryo survival and could have implications for improving human oocyte culture techniques.
COMPETING INTERESTS
The author(s) declare that they have no competing interests.
AUTHORS' CONTRIBUTIONS
Lien Boi Linh Nguyen: Writing – original draft, Methodology, Investigation. Ba Anh My Le: Writing – original draft, Methodology, Investigation. Chi Thien Lam: Methodology, Investigation, Formal analysis. Ngoc Thao Vy Nguyen: Investigation, Methodology, Formal analysis. Nhat-Thinh Nguyen: Formal analysis, Investigation, Methodology. Van Thuan Nguyen: Funding acquisition, Project administration, Supervision, Writing – review & editing. Hong-Thuy Bui: Writing – review & editing, Supervision, Project administration, Methodology, Funding acquisition, Formal analysis.
ACKNOWLEDGEMENTS
This research is funded by Vietnam National University Ho Chi Minh City (VNU-HCM) under Grant No. B2022-28-01.

